Large interfacial area enhances electrical conductivity of poly(3-hexylthiophene)/insulating polymer blends†
Jiayue Chenabc,
Zhaobin Chena,
Yunpeng Qua,
Guanghao Lua,
Feng Yeabc,
Sisi Wangabc,
Hongying Lvabc and
Xiaoniu Yang*ab
aPolymer Composites Engineering Laboratory, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Renmin Street 5625, Changchun 130022, P. R. China. E-mail: xnyang@ciac.ac.cn; Fax: +86-43185262028; Tel: +86-43185262206
bState Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Renmin Street 5625, Changchun 130022, P. R. China
cUniversity of Chinese Academy of Sciences, 19 Yuquan Rd., Shijingshan District, Beijing 100049, P. R. China
First published on 20th November 2014
Abstract
Semiconducting polymer/insulating polymer blends are promising materials for applications in organic optoelectronics. Here, two semiconducting polymer (SP)/insulating polymer (IP) blends, poly(3-hexylthiophene) (P3HT) and polystyrene (PS) or polyisoprene (PI), were prepared. The relationship between the electrical conductivity and the crystallinity/morphology of the P3HT/IP blends was systematically investigated. To prepare P3HT/IP blend films with different morphology, in particular the length-scale of phase separation, a P3HT/IP solution was obtained via mixing P3HT and IP solutions. The mixing homogeneity between P3HT and IP in the solution was controlled via tuning mixing time of the two solutions. The conductivity of the P3HT/IP blend film significantly increased with mixing time, as a consequence of decreased scale of phase separation between P3HT and IP. We therefore conclude that this enhanced conductivity of the blends is attributed to the large interfacial area between these two components of the blend, rather than crystallinity of P3HT, which presents a downward trend with increasing time. The relationship between the electrical properties and the interfacial area provides SP/IP blends with an important criterion both for scientific research and real application.
Introduction
Organic semiconductors have attracted increasing interest in recent decades1–5 because of their advantages of flexibility, light weight, and easy fabrication for large area devices via low-temperature solution processing compared with amorphous silicon. However, there is still plenty scope to further reduce cost, and improve stability and device performance to meet requirements for industrial application. To this end, various technologies, including copolymerization and blending, have been developed and intensively studied in the past ten years.6−12 It has been found that copolymerizing or blending semiconducting polymers (SP) with insulating polymers (IP) not only decreases cost and enhances stability, but also improves the electrical properties of organic semiconducting polymers, and different mechanisms have been proposed for this.10,11,13–24 Arias,10 Qiu,21 and Kumar16 reported that when blending P3HT (or poly[5,5′-bis(3-dodecyl-2-thienyl)-2,2′-bithiophene] (PQT-12)) with polyethylene (PE) or poly(methyl methacrylate) (PMMA), the carrier mobilities of the SP/IP blends were higher than those of the pure semiconducting polymers, which could be ascribed to the vertical phase separation structure and the formation of a continuous semiconductor phase in the channel, benefiting charge transport. Improved transistor performance was also observed in other SP/IP blends by Qiu12 and Yu,24 and the higher performance of the devices was considered mainly to result from the high crystallinity and molecular ordering of the semiconducting polymers. Recently, Lu observed that the field-effect mobility of P3HT could be enhanced by oxygen-induced moderate doping.18 Other mechanisms have been proposed, for example, “zone-refinement effect” (for triethylsilylethynyl anthradithiophene (TESADT) and poly(α-methylstyrene) (PαMS) system),13 but, to date, the mechanism for charge transport in SP/IP blends or copolymers is still not very clear.Compared with the copolymerization method, physical blending is cost-effective and easy to perform. Therefore, in our previous work, a series of blends were prepared of poly(3-butylthiophene) (P3BT) with crystalline and amorphous IP matrix, including poly(ethylene oxide) (PEO) and amorphous-polystyrene (a-PS).19 It was found that conductivity of the P3BT/PEO blend was far lower than that of the P3BT/a-PS blend, which was thought to correlate with the smaller interfacial area between P3BT and PEO phases resulting from formation of bundled P3BT cluster under the crystallization of PEO. However, because PEO and a-PS are polymers with different physical properties, the influence of IPs themselves on the electrical performance of P3BT/IP blends could not be excluded.
To determine the exact relationship between interfacial area and conductivity of SP/IP blends, in this work, two amorphous insulating polymers, PS and polyisoprene (PI), were selected to blend with P3HT, and the conductivity was first investigated as a function of composition and film thickness. Furthermore, through precisely designing the experimental route and carefully controlling the conditions, different morphologies were obtained for P3HT/IP blend films with the same composition (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio) and comparable thickness (around 80 nm). It was found that the enhanced conductivity of P3HT/IP blends is directly correlated to the interfacial area between P3HT and IP phases, rather than crystallinity of P3HT.
1 weight ratio) and comparable thickness (around 80 nm). It was found that the enhanced conductivity of P3HT/IP blends is directly correlated to the interfacial area between P3HT and IP phases, rather than crystallinity of P3HT.
Experimental section
Materials
The semiconducting polymer, regioregular P3HT (Mw = 47 kg mol−1, PDI = 1.72), was synthesized according to methods described in a previous study.25 The insulating polymer, amorphous PI rubber (Mw = 396 kg mol−1, PDI = 2.53), was synthesized following a procedure reported in the literature,26 and amorphous PS (Mw = 124 kg mol−1, PDI = 1.64) was obtained from DaQing Petro Chemical Company. o-Dichlorobenzene (ODCB, anhydrous, 99%) and dioxane were purchased from Sigma-Aldrich Co. and Beijing Chemical Reagent Co. Ltd, China, respectively. All these materials were used as received without further purification.Sample preparation
11.4 mg mL−1 of P3HT solution in ODCB was prepared by heating at 70 °C for 1 h, and then poor solvent dioxane (ODCB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dioxane = 7
dioxane = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume) was added to the solution. The solution was kept at 70 °C to obtain a homogeneous solution with concentration of 10 mg mL−1, and then aged at 20 °C in a dark and still environment for 2 days to allow the P3HT molecules to crystallize/self-organize. A solution of insulating polymer (PS or PI) with a concentration of 10 mg mL−1 in ODCB/dioxane solvent mixture was prepared following the same procedure. The freshly obtained insulating polymer solution was then mixed with the aged P3HT solution, and the blend solution was agitated and sonicated for 2 min. After mixing for different time at 20 °C, the P3HT/IP solution was spin-coated (Laurell Spin Processor WS-400B 6NPP Lite) onto a pre-cleaned glass slide or patterned indium tin oxide (ITO) glass, and the film thickness was controlled in the range of 60–180 nm by adjusting the spin-coating conditions (speed, accelerated velocity, and time). To study the relationship between conductivity and film thickness, thicker films (0.8–4 μm) were prepared using a drop-casting technique.
1 by volume) was added to the solution. The solution was kept at 70 °C to obtain a homogeneous solution with concentration of 10 mg mL−1, and then aged at 20 °C in a dark and still environment for 2 days to allow the P3HT molecules to crystallize/self-organize. A solution of insulating polymer (PS or PI) with a concentration of 10 mg mL−1 in ODCB/dioxane solvent mixture was prepared following the same procedure. The freshly obtained insulating polymer solution was then mixed with the aged P3HT solution, and the blend solution was agitated and sonicated for 2 min. After mixing for different time at 20 °C, the P3HT/IP solution was spin-coated (Laurell Spin Processor WS-400B 6NPP Lite) onto a pre-cleaned glass slide or patterned indium tin oxide (ITO) glass, and the film thickness was controlled in the range of 60–180 nm by adjusting the spin-coating conditions (speed, accelerated velocity, and time). To study the relationship between conductivity and film thickness, thicker films (0.8–4 μm) were prepared using a drop-casting technique.
Sample films for transmission electron microscopy (TEM) investigation were obtained by a floating method, in which the thin films were immersed in saturated vapor of hydrofluoric acid for 3 s, floated onto the surface of deionized water, and then transferred to copper grids.
Sample powders collected from the spin-coated films were used for thermal analysis. For the powder X-ray diffraction (XRD) characterization, the powders were pressed into a thin square slice sized 1.1 cm × 0.8 cm, and a square slice of pure PS was also prepared and scanned to eliminate the background signals.
Characterization
The substrate of the sample films for conductivity measurement was the etched ITO glass with a channel 300 μm wide. Considering the low conductivity values of the polymer films relative to the ITO electrodes, contact resistance of the probes is negligible,27 so a two-probe method was adopted with ITO as both electrodes. The measurement was carried out with a Keithley-2400 Source Meter in air 24 h after the films were fabricated, and conductivity was calculated according to Ohm's law (eqn (1)):| σ = Iw/Vdl | (1) |
Optical microscopy (OM) investigations were carried out using a Carl Zeiss A1m microscope equipped with an Infinity 4-11 Digital Camera (Lumenera Co., Canada).
TEM was performed on a JEOL JEM-1011 transmission electron microscope operated at an acceleration voltage of 100 kV. The samples were dried at room temperature for more than 12 h before measurement.
Surface morphology of the films was observed on an Agilent 5500 AFM by tapping mode in an ambient atmosphere.
UV-Vis absorption spectra were acquired on a Lambda 750 spectrometer (PerkinElmer, Wellesley, MA).
Powder XRD was conducted on Rigaku D/MAX-2500 using Cu Kα1 radiation with X-ray generation power of 40 kV tube voltage and 200 mA tube current. The scan was performed at 2θ/θ scanning mode. For all the samples, the scanning speed was 0.1° (2θ) per minute with 0.02° (2θ) step size.
Thermal analysis was performed on TA Q100 DSC at 10 °C min−1 heating rate under a nitrogen atmosphere, and three replicates were made for each sample.
Thickness of the film was measured on a KLA-Tencor D-100 surface profiler.
The rheological properties of the P3HT/PS solution were measured with a Wells/Brookfield DV-III ultra rheometer equipped with a constant temperature bath. The solution (0.5 mL) was poured into a sample cup and the shear viscosity was measured using an established program. The measurement was carried out at a shear rate of 150 S−1 at 20 °C.
Results and discussion
It is well recognized that both the composition and film thickness of copolymers or polymer blends strongly correlate with their electrical performance.15,28–31 Therefore, in this study, the effect of the composition and the film thickness on electrical conductivity of a P3HT/IP blend was first investigated before further experiment. Fig. 1a illustrates the dependence of the conductivity on composition of spin-coated P3HT/PS (70 nm) and P3HT/PI (80 nm) blend films, and clearly shows that the conductivity first increases, reaches maximum, and then decreases with decreasing P3HT content. Regardless of the insulating polymer used, its addition substantially enhances the conductivity of pure P3HT (1.8 × 10−4 S cm−1), unless the blend film has over 90 wt% of insulating matrix. Although the conductivity of P3HT/PI is higher than that of P3HT/PS in the whole range, the highest values for both P3HT/PI (15.5 × 10−4 S cm−1) and P3HT/PS (7.1 × 10−4 S cm−1) blends are obtained at the composition of 50 wt% P3HT/50 wt% IP, which is consistent with our previous work.20 | ||
| Fig. 1 (a) Dependence of conductivity on composition of spin-coated P3HT/PS (70 nm) and P3HT/PI (80 nm) blend films. (b) Dependence of conductivity on film thickness of 50 wt% P3HT/50 wt% PS blends. | ||
The relationship between the conductivity and film thickness is shown in Fig. 1b. It can be seen that although the blend films were individually prepared by different techniques, a general trend of monotonic decrease in the conductivity with increasing film thickness could be clearly observed. For example, for spin-coated P3HT/PS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio) film, the conductivity dramatically decreases from 7.0 × 10−4 S cm−1 for the 70 nm thick film to 4.8 × 10−4 S cm−1 for the film with thickness of 160 nm; after that it decreases slowly. The drop-cast P3HT/PS film exhibits the same variation although the film thickness is much thicker (inset in Fig. 1b), and it reaches a stable value (around 1.6 × 10−4 S cm−1) when the film is up to 3 μm thick. Actually, the P3HT/PI blend film shows the same variation (Fig. S1†).
1 weight ratio) film, the conductivity dramatically decreases from 7.0 × 10−4 S cm−1 for the 70 nm thick film to 4.8 × 10−4 S cm−1 for the film with thickness of 160 nm; after that it decreases slowly. The drop-cast P3HT/PS film exhibits the same variation although the film thickness is much thicker (inset in Fig. 1b), and it reaches a stable value (around 1.6 × 10−4 S cm−1) when the film is up to 3 μm thick. Actually, the P3HT/PI blend film shows the same variation (Fig. S1†).
The effect of film thickness on electrical properties of organic devices has been reported by other research groups.32–34 However, to the best of our knowledge, this study is the first to demonstrate the monotonic decrease in in-plane conductivity with increasing film thickness. We suggest that the orientation of the P3HT nanowhiskers in blend film is responsible for the conductivity variation.35 When the film is thin, the P3HT nanowhiskers mainly orient within the film plane, and the applied electrical field is just along this direction, so the conductivity is high. As the film thickness increases, the P3HT fibers align randomly, and the blend film is much more like a three-dimensional bulk material. Consequently, the portion of the fibers orienting in the plane decreases, which results in lower film conductivity. This is evidenced by the out-of-plane conductivity of the film with thickness of 140 nm (in the order of 10−7 S cm−1) being far less than the in-plane conductivity (5 × 10−4 S cm−1). To clearly observe the conductivity difference and reconcile the contribution from the film thickness, the following describes blend films prepared with comparable thickness (around 80 nm) at a fixed composition (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio).
1 weight ratio).
The conductivity of the spin-coated P3HT/PS blend film is unexpectedly related to the mixing time of P3HT/PS solution before spin-coating. The conductivity of P3HT/PS blend film is greatly enhanced by mixing for a longer time, and the optical microscopy characterization (Fig. 2) shows that longer mixing time promotes formation of much more homogeneous blend film. Therefore, it is assumed that the enhanced conductivity is caused by the larger interfacial area between P3HT and PS phases. (It is noteworthy that the P3HT/PS blend actually consists of P3HT crystal whiskers, amorphous P3HT domains, and PS component, in which the P3HT crystal whiskers serve as the basic charge transport pathway, and the interface related to the enhanced conductivity is referred to the interface between P3HT crystal whiskers and PS component. Therefore, the terminology of “P3HT crystal whisker/P3HT/PS blend” or “P3HT crystal whisker/P3HT/PI blend” is more accurate. However, the term “P3HT/PS blend” or P3HT/PI blend” is used throughout the text for concision.) To confirm this assumption, we precisely designed the experiment route and carefully controlled the operation conditions, and finally established the relationship between interfacial area and the conductivity of P3HT/IP blend films.
 | ||
Fig. 2 OM images of P3HT/PS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) blend films spin-coated from solution mixing for different time: (a) 0 h, (b) 1 h, (c) 3 h, and (d) 6 h. 1) blend films spin-coated from solution mixing for different time: (a) 0 h, (b) 1 h, (c) 3 h, and (d) 6 h. | ||
Fig. 3 shows the TEM images of P3HT/PS blend films spin-coated from solution mixing for different time. In these TEM images, the P3HT-rich domains appear dark because of its crystalline feature and relatively higher density than PS, whereas the bright portion is amorphous PS-rich domain. From Fig. 3 it can be seen that the P3HT/PS blend film shows the morphology with large-scale phase separation structure, in which the insulating amorphous PS component is the continuous phase, while the P3HT-rich domain is isolated, if the mixing time is less than 3 h. As the mixing time increases, the irregularly dispersed P3HT aggregates become smaller, decreasing from about 4–5 μm (Fig. 3a) diameter, through 1–2 μm (Fig. 3b) to less than 1 μm (Fig. 3c). Under careful observation, it is found that the P3HT and PS phases are not totally separated, and numerous long P3HT fibers (width of about 15 nm) penetrate throughout the bright PS phase, although most densely overlap with each other and aggregate to form the P3HT-rich phase (Fig. 4). By extending the mixing time, the P3HT/PS blend films tend to form much more homogeneous morphology. As shown in Fig. 3d, when the time reaches 6 h, the boundary between P3HT and PS components disappears, and the large-scale phase separation structure can barely be seen (although a few P3HT aggregates can be found with 300 nm diameter). The homogeneity of the blend film gets better when time is prolonged to 19 h (Fig. 3e), and finally, a P3HT/PS blend film with homogeneous interpenetration network morphology is obtained for the solution mixing for 24 h (Fig. 3f). In comparison with the pure P3HT film (Fig. 3g, inset in Fig. 3f), in which the P3HT whiskers are highly aggregated, it is found that the P3HT crystal whiskers are well dispersed without severe aggregations in P3HT/PS blend, which could be ascribed to the role of the PS as an efficient dispersing agent. The morphological evolution of the film is believed to arise from the distribution of two components in solution: after mixing, the P3HT molecules or fibers and the PS molecules interdiffused into each other because of a thermodynamic effect; as the mixing time increased, the interdiffusion process proceeded sufficiently, and more uniform solution was formed. The uniform solution was finally fixed to form homogeneous morphology in the blend film during the spin-coating process. The distribution status of two components in solution was verified indirectly via the rheological measurement, as shown in Fig. 5. It can be seen that the viscosity of P3HT/PS solution dramatically increases with mixing time and flattens at around 27 h, which is believed to result from the more uniform distribution and mutual entanglement of P3HT fibers and PS molecules. Additionally, the density of dispersed P3HT fibers immersed in the amorphous PS matrix increases with time because of the development of P3HT aggregates into fibers. All the phenomena described above result in larger and larger interfacial area between P3HT and PS phases.
 | ||
| Fig. 4 (a) AFM images of P3HT/PS blend film spin-coated from solution mixing for 1 h. (b) Magnified images for the area marked in (a). | ||
From the above discussions, it is found that as mixing time increases, the blend solution becomes uniform and, after spin-coating, more homogeneous blend film with larger interfacial area between P3HT and PS is formed. As expected, the electrical conductivity of P3HT/PS blend film gradually rises with mixing time (Fig. 6). It is 3.3 × 10−4 S cm−1 for 0 h film, which significantly increases to about 5.5 × 10−4 S cm−1 at 3 h. After that, the conductivity maintains the gradually increasing trend, and reaches 6.5 × 10−4 S cm−1 with 24 h of mixing time.
The same phenomenon was observed for the P3HT and polyisoprene (PI) blend. PI, as well as PS, studied above, is an amorphous polymer with low dielectric constant ε (εPS = 2.49–2.55, εPI = 2.37–2.45),36 which could minimize the impact on electrical conductivity caused by dipolar disorder.14,31 Fig. S2(a)† shows the TEM images of P3HT/PI blend film spin-coated from the solution mixing for different time. It is found that, similar to P3HT/PS film (Fig. 3), the P3HT/PI blend film shows more homogeneous morphology with increasing mixing time, and long P3HT fibers are dispersed within the whole film. Fig. S2(b)† shows that as the morphology evolves, the conductivity of P3HT/PI blend film displays the same increasing trend.
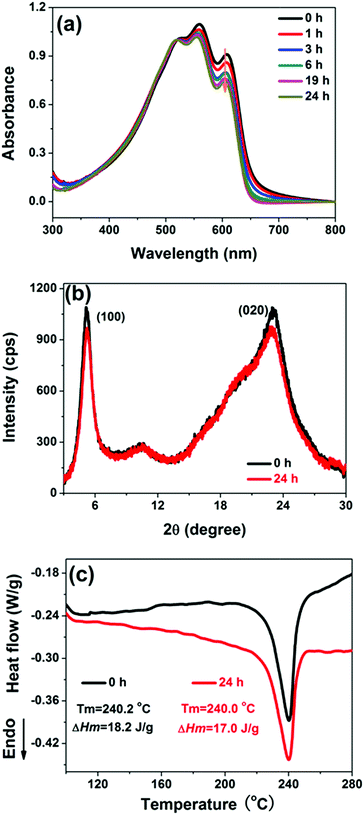
In general, the degree of molecular ordered arrangement is one of the most key parameters that directly influence the electrical properties of organic semiconductors.22,37–40 In the present study, this aspect was also taken into consideration. As the PS (or PI) component has no obvious absorption features in the wavelength range recorded (Fig. S3†), the spectra of P3HT/PS or P3HT/PI blend films were used to represent the absorption of P3HT. As shown by UV-Vis absorption spectra in Fig. 7a, three absorption peaks could be observed at 520 nm, 560 nm, and 605 nm for P3HT. Among them, the last (605 nm) is associated with an interchain absorption and its intensity is correlated with the degree of interchain order.39,41,42 Overall, as the mixing time before spin-coating is prolonged, the intensities of the peaks successively decrease, indicating lower crystallinity. For P3HT/PI blend films, the crystallinity of P3HT also decreases with increasing mixing time (Fig. S4†).
The variation in crystallinity with increasing mixing time is further corroborated by XRD and DSC analyses, and Fig. 7b and c shows the corresponding curves of P3HT/PS blend films mixing for 0 h and 24 h before spin-coating, respectively, as typical examples. In the XRD profiles, two peaks could be resolved for both sample films. The first, at around 2θ = 5.2°, represents the crystallographic (100) of P3HT, while the peak at around 2θ = 23.0° is assigned to the reflection of the crystallographic (020) plane of P3HT crystals.43,44 The stronger peak intensity indicates higher crystallinity of P3HT/PS blend film with 0 h of mixing time. That the crystallinity of the film with shorter mixing time is higher could also be reflected from the DSC heating curves. In comparison with the blend film mixing for 24 h, the 0 h sample film not only shows the relatively higher melting point (240.2 °C vs. 240.0 °C), but also higher melting enthalpy (18.2 J g−1 vs. 17.0 J g−1), and the corresponding crystallinity is 18.4% and 17.2%, respectively, determined by Xc(%) = (ΔHm/ΔHm*) × 100%(ΔHm* = 99 J g−1).45 Combining the above results of UV-Vis, powder XRD, and DSC experiments, it can be concluded that the crystallinity of the blend films decreases as the mixing time is prolonged.
Generally speaking, the crystallinity of the solution increases with the aging time.39,46 The opposite phenomenon in this work is mainly a result of the unique process of solution mixing and film deposition. Taking the P3HT/PS system for example, because the P3HT had already sufficiently crystallized into whiskers with lengths of hundreds of nanometers to several micrometers and widths of 15 nm in the solution (Fig. S5†) before mixing with PS solution of the same concentration, the mixing step could be regarded as a dilution and dissolution process at the very beginning. In other words, after mixing with PS solution, the individual P3HT concentration in the blend solution was reduced, and some of the already formed imperfect P3HT crystals gradually dissolved, therefore, the P3HT crystallinity decreased. Moreover, it has been well-documented that the crystallization of a polymer component in its blend (or composite) is usually suppressed compared with that of its pure bulk state.47,48 The presence of PS would suppress the crystallization of those nonaggregated fractions of P3HT during the film deposition process. As the mixing time increases, the two components distribute homogeneously in solution; during casting films, the scale of liquid–liquid phase separation becomes small, so the inhibiting effect becomes obvious. The two aspects described above lead to gradually decreased crystallinity of final blend films. To the best of our knowledge, higher crystallinity of conjugated polymer is of benefit for enhancing charge transport; at least, this would not cause negative impact on charge transport.14,23,39,40,49 While in this case, as the mixing time increases, the crystallinity of P3HT/PS blend film decreases, which is inconsistent with the conductivity increase. In other words, the changes in electrical conductivity could not be attributed to crystallinity variation.
For conjugated polymers or their blends, the morphology of conjugated polymers themselves is also critical to performance.22,50 Serving as one of the most typical conjugated polymers, P3HT could form various forms of crystallites with different capability to transport charge carriers. In our case, the long P3HT fibers were generated in P3HT solution before mixing with PS solution. This morphology of the P3HT fibers was frozen not only in P3HT aggregates (Fig. 4), but also in the PS phase (Fig. 3a) after spin-coating process. In other words, the P3HT fibers, which carry the charge transport in P3HT/PS blend film, are unchanged in terms of morphology for all samples. In addition, the distribution of P3HT fibers must be taken into consideration. The conductivity will be lowered if few P3HT fibers are distributed in the insulating PS matrix, because of the lack of ability to form continuous conducting pathways. However, once the continuous conducting pathways are constructed, the conductivity would not depend on either the amount of the conducting polymer in the blend or the distribution of the conducting polymer in the insulating matrix. For example, our previous work reported that for P3BT/PS blend with homogeneous morphology (P3BT fibers evenly distributed in PS matrix), the conductivity of the blend shows the highest value in the range of 40–60 wt% PS, and is higher than that of pure P3BT as long as the amount of P3BT in the blend is above 10 wt%.20 In the present study, a similar phenomenon was also found for P3HT/PS or P3HT/PI blends (Fig. 1a). In other words, the continuous conducting pathway has already been constructed in all cases. Otherwise, the conductivity of the blend will show a monotonic decrease on increasing the amount of insulating polymer in the blend. This is further demonstrated by the morphologies of P3HT/PS (or P3HT/PI) blends shown in Fig. 3 and S2(a),† in which numerous P3HT fibers interpenetrate the whole film and provide the basic carrier transportation pathways. Therefore, it is safe to conclude that the conductivity change for the P3HT/IP blend could not be caused by the morphology and distribution of the semiconducting P3HT in the blend.
Excluding the influence of crystallinity and morphology of the P3HT component, it is believed that the enhanced electrical conductivity of the P3HT/IP blend film mainly results from the large interfacial area between P3HT and IP components. From the discussion above it is known that the P3HT continuous conducting pathways have already been constructed in the whole film, which is the important prerequisite for the enhanced conductivity. However, the carrier (e.g. polaron) transportation is still affected by the local environment surrounding the P3HT fibers.51,52 Serving as the matrix in the blend, the a-PS (or PI) has low polarizability, indicating weaker carrier–matrix interaction. Correspondingly, the hindrance of charge transportation at the P3HT/IP interface is considerably reduced, which leads to reduced activation energy and higher carrier mobility in comparison with that of pure P3HT.19 Therefore, enhanced conductivity is obtained, compared with the pure P3HT (Fig. 1a). As the mixing time increases, the morphology of the P3HT/PS blend films (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio) becomes much more homogeneous and the interfacial area between the two phases increases. As a result, the electrical conductivity of the P3HT/IP blend film increases with time. It is worth noting that mixing P3HT with PS at a molecular level, which will lead to the largest interfacial area, is not realistic because of the immiscibility between P3HT and PS. The significant contribution of interfacial area to the electrical performance of the blend has overwhelmed the negative effect of slightly decreased crystallinity of P3HT. The relationship between electrical conductivity and the interfacial area of P3HT/PS and P3HT/PI blends is believed to be applicable to other SP/IP blend systems, which provides an important criterion for promoting the electrical performance of devices based on semiconducting polymer/insulating polymer blends.
1 weight ratio) becomes much more homogeneous and the interfacial area between the two phases increases. As a result, the electrical conductivity of the P3HT/IP blend film increases with time. It is worth noting that mixing P3HT with PS at a molecular level, which will lead to the largest interfacial area, is not realistic because of the immiscibility between P3HT and PS. The significant contribution of interfacial area to the electrical performance of the blend has overwhelmed the negative effect of slightly decreased crystallinity of P3HT. The relationship between electrical conductivity and the interfacial area of P3HT/PS and P3HT/PI blends is believed to be applicable to other SP/IP blend systems, which provides an important criterion for promoting the electrical performance of devices based on semiconducting polymer/insulating polymer blends.
Conclusions
Two series of semiconducting polymer (SP)/insulating polymer (IP) blend films, including P3HT/PS and P3HT/PI, were systematically investigated in this study, which shows that the conductivity of the P3HT/IP blend films is higher than that of pure P3HT as long as the P3HT amount is above 10 wt%, and reaches maximum at a composition of 50 wt% P3HT/50 wt% IP; moreover, the conductivity of P3HT/IP blends monotonically decreases with increasing film thickness up to 3 μm. By tuning the mixing time of the blend solution before spin-coating, P3HT/IP blend films (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio, around 80 nm thick) with different morphologies were obtained. TEM and AFM images show that, as the mixing time increases, the morphologies of the blend films gradually change from a large-scale phase separation structure to a homogeneous interpenetrating network structure, indicating a larger interfacial area between P3HT and PS (or PI). The dramatically increasing trend with time is also observed for the conductivity of P3HT/IP blend films. Considering that the decreased P3HT crystallinity could not impact positively on the conductivity, it is concluded that the significantly enhanced conductivity is beneficial from the increase in the interfacial area. The relationship between the conductivity and interfacial area of SP/IP blends explored in this work could play an important role in research and development of cost-effective, robust, and environmentally stable all-polymer blends for organic electronics.
1 weight ratio, around 80 nm thick) with different morphologies were obtained. TEM and AFM images show that, as the mixing time increases, the morphologies of the blend films gradually change from a large-scale phase separation structure to a homogeneous interpenetrating network structure, indicating a larger interfacial area between P3HT and PS (or PI). The dramatically increasing trend with time is also observed for the conductivity of P3HT/IP blend films. Considering that the decreased P3HT crystallinity could not impact positively on the conductivity, it is concluded that the significantly enhanced conductivity is beneficial from the increase in the interfacial area. The relationship between the conductivity and interfacial area of SP/IP blends explored in this work could play an important role in research and development of cost-effective, robust, and environmentally stable all-polymer blends for organic electronics.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant nos 20925415 and 20990233), the Solar Energy Initiative (Grant no. KGCX2-YW-399+9) of the Chinese Academy of Sciences, and the Hi-Tech Research and Development Program (863) of China (2011AA050524).Notes and references
- G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Science, 1995, 270, 1789–1791 CAS.
- H. Sirringhaus, N. Tessler and R. H. Friend, Science, 1998, 280, 1741–1744 CrossRef CAS.
- H. E. Katz, Chem. Mater., 2004, 16, 4748–4756 CrossRef CAS.
- A. C. Arias, J. D. MacKenzie, I. McCulloch, J. Rivnay and A. Salleo, Chem. Rev., 2010, 110, 3–24 CrossRef CAS PubMed.
- A. Pron, P. Gawrys, M. Zagorska, D. Djurado and R. Demadrille, Chem. Soc. Rev., 2010, 39, 2577–2632 RSC.
- G. Ren, P.-T. Wu and S. A. Jenekhe, Chem. Mater., 2010, 22, 2020–2026 CrossRef CAS.
- B. J. Campo, D. Bevk, J. Kesters, J. Gilot, H. J. Bolink, J. Zhao, J. C. Bolsee, W. D. Oosterbaan, S. Bertho, J. D'Haen, J. Manca, L. Lutsen, G. Van Assche, W. Maes, R. A. J. Janssen and D. Vanderzande, Org. Electron., 2013, 14, 523–534 CrossRef CAS PubMed.
- P. P. Khlyabich, B. Burkhart and B. C. Thompson, J. Am. Chem. Soc., 2012, 134, 9074–9077 CrossRef CAS PubMed.
- L. Yang, H. Zhou, S. C. Price and W. You, J. Am. Chem. Soc., 2012, 134, 5432–5435 CrossRef CAS PubMed.
- A. C. Arias, F. Endicott and R. A. Street, Adv. Mater., 2006, 18, 2900–2904 CrossRef CAS.
- T. A. M. Ferenczi, C. Mueller, D. D. C. Bradley, P. Smith, J. Nelson and N. Stingelin, Adv. Mater., 2011, 23, 4093–4097 CrossRef CAS PubMed.
- L. Z. Qiu, W. H. Lee, X. H. Wang, J. S. Kim, J. A. Lim, D. Kwak, S. Lee and K. Cho, Adv. Mater., 2009, 21, 1349–1353 CrossRef CAS.
- Y. S. Chung, N. Shin, J. Kang, Y. Jo, V. M. Prabhu, S. K. Satija, R. J. Kline, D. M. DeLongchamp, M. F. Toney, M. A. Loth, B. Purushothaman, J. E. Anthony and D. Y. Yoon, J. Am. Chem. Soc., 2011, 133, 412–415 CrossRef CAS PubMed.
- S. Goffri, C. Muller, N. Stingelin-Stutzmann, D. W. Breiby, C. P. Radano, J. W. Andreasen, R. Thompson, R. A. J. Janssen, M. M. Nielsen, P. Smith and H. Sirringhaus, Nat. Mater., 2006, 5, 950–956 CrossRef CAS PubMed.
- F. S. Kim and S. A. Jenekhe, Macromolecules, 2012, 45, 7514–7519 CrossRef CAS.
- A. Kumar, M. A. Baklar, K. Scott, T. Kreouzis and N. Stingelin-Stutzmann, Adv. Mater., 2009, 21, 4447–4451 CrossRef CAS.
- J.-Y. Lee, C.-J. Lin, C.-T. Lo, J.-C. Tsai and W.-C. Chen, Macromolecules, 2013, 46, 3005–3014 CrossRef CAS.
- G. Lu, J. Blakesley, S. Himmelberger, P. Pingel, J. Frisch, I. Lieberwirth, I. Salzmann, M. Oehzelt, R. Di Pietro, A. Salleo, N. Koch and D. Neher, Nat. Commun., 2013, 4, 1588 CrossRef PubMed.
- G. Lu, H. Tang, Y. Huan, S. Li, L. Li, Y. Wang and X. Yang, Adv. Funct. Mater., 2010, 20, 1714–1720 CrossRef CAS.
- G. Lu, H. Tang, Y. Qu, L. Li and X. Yang, Macromolecules, 2007, 40, 6579–6584 CrossRef CAS.
- L. Qiu, J. A. Lim, X. Wang, W. H. Lee, M. Hwang and K. Cho, Adv. Mater., 2008, 20, 1141–1145 CrossRef CAS.
- L. Qiu, X. Wang, W. H. Lee, J. A. Lim, J. S. Kim, D. Kwak and K. Cho, Chem. Mater., 2009, 21, 4380–4386 CrossRef CAS.
- J. Sun, B. J. Jung, T. Lee, L. Berger, J. Huang, Y. Liu, D. H. Reich and H. E. Katz, ACS Appl. Mater. Interfaces, 2009, 1, 412–419 CAS.
- X. Yu, K. Xiao, J. Chen, N. V. Lavrik, K. Hong, B. G. Sumpter and D. B. Geohegan, ACS Nano, 2011, 5, 3559–3567 CrossRef CAS PubMed.
- R. S. Loewe, S. M. Khersonsky and R. D. McCullough, Adv. Mater., 1999, 11, 250–253 CrossRef CAS.
- G. Ricci, D. Morganti, A. Sommazzi, R. Santi and F. Masi, J. Mol. Catal. A: Chem., 2003, 204, 287–293 CrossRef.
- K.-H. Yim, G. L. Whiting, C. E. Murphy, J. J. M. Halls, J. H. Burroughes, R. H. Friend and J.-S. Kim, Adv. Mater., 2008, 20, 3319–3324 CrossRef CAS.
- S.-W. Liu, C.-C. Lee, H.-L. Tai, J.-M. Wen, J.-H. Lee and C.-T. Chen, ACS Appl. Mater. Interfaces, 2010, 2, 2282–2288 CAS.
- J. S. Liu, E. Sheina, T. Kowalewski and R. D. McCullough, Angew. Chem., Int. Ed., 2002, 41, 329–332 CrossRef CAS.
- J. Lee, K. Kim, J. H. Kim, S. Im and D. Y. Jung, Appl. Phys. Lett., 2003, 82, 4169–4171 CrossRef CAS PubMed.
- A. Babel and S. A. Jenekhe, Macromolecules, 2004, 37, 9835–9840 CrossRef CAS.
- W. Li, K. H. Hendriks, W. S. C. Roelofs, Y. Kim, M. M. Wienk and R. A. J. Janssen, Adv. Mater., 2013, 25, 3182–3186 CrossRef CAS PubMed.
- S. Joshi, S. Grigorian, U. Pietsch, P. Pingel, A. Zen, D. Neher and U. Scherf, Macromolecules, 2008, 41, 6800–6808 CrossRef CAS.
- L. Resendiz, M. Estrada, A. Cerdeira, B. Iniguez and M. J. Deen, Org. Electron., 2010, 11, 1920–1927 CrossRef CAS PubMed.
- B. Huang, E. Glynos, B. Frieberg, H. Yang and P. F. Green, ACS Appl. Mater. Interfaces, 2012, 4, 5204–5210 CAS.
- I. Furuta, S. I. Kimura, M. Iwama and D. Schrader, in Polymer Handbook, ed. J. Brandrup, E. H. Immergut and E. A. Grulke, Wiley-Interscience Publication, New York, 4th edn, 1998, ch. V, pp. 5 and 92 Search PubMed.
- Y. D. Park, H. S. Lee, Y. J. Choi, D. Kwak, J. H. Cho, S. Lee and K. Cho, Adv. Funct. Mater., 2009, 19, 1200–1206 CrossRef CAS.
- H. Li, H. Tang, L. Li, W. Xu, X. Zhao and X. Yang, J. Mater. Chem., 2011, 21, 6563–6568 RSC.
- L. Li, G. Lu and X. Yang, J. Mater. Chem., 2008, 18, 1984–1990 RSC.
- B. J. Kim, H. S. Lee, J. S. Lee, S. Cho, H. Kim, H. J. Son, M. J. Ko, S. Park, M. S. Kang, S. Y. Oh, B. Kim and J. H. Cho, J. Phys. Chem. C, 2013, 117, 11479–11486 CAS.
- P. J. Brown, D. S. Thomas, A. Kohler, J. S. Wilson, J. S. Kim, C. M. Ramsdale, H. Sirringhaus and R. H. Friend, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 67, 064203 CrossRef.
- H. G. O. Sandberg, G. L. Frey, M. N. Shkunov, H. Sirringhaus, R. H. Friend, M. M. Nielsen and C. Kumpf, Langmuir, 2002, 18, 10176–10182 Search PubMed.
- H. Sirringhaus, P. J. Brown, R. H. Friend, M. M. Nielsen, K. Bechgaard, B. M. W. Langeveld-Voss, A. J. H. Spiering, R. A. J. Janssen, E. W. Meijer, P. Herwig and D. M. de Leeuw, Nature, 1999, 401, 685–688 CrossRef CAS PubMed.
- S. Nam, M. Shin, H. Kim and Y. Kim, Nanoscale, 2010, 2, 2384–2389 RSC.
- S. Malik and A. K. Nandi, J. Polym. Sci., Part B: Polym. Phys., 2002, 40, 2073–2085 CrossRef CAS.
- W. Xu, H. Tang, H. Lv, J. Li, X. Zhao, H. Li, N. Wang and X. Yang, Soft Matter, 2012, 8, 726–733 RSC.
- X. Yang, G. Lu, L. Li and E. Zhou, Small, 2007, 3, 611–615 CrossRef CAS PubMed.
- P. Vanlaeke, A. Swinnen, I. Haeldermans, G. Vanhoyland, T. Aernouts, D. Cheyns, C. Deibel, J. D'Haen, P. Heremans, J. Poortmans and J. V. Manca, Sol. Energy Mater. Sol. Cells, 2006, 90, 2150–2158 CrossRef CAS PubMed.
- S. Li, S. wang, B. Zhang and X. Yang, Chin. J. Appl. Chem., 2014, 31, 20–24 CAS.
- C. Muller, S. Goffri, D. W. Breiby, J. W. Andreasen, H. D. Chanzy, R. A. J. Janssen, M. M. Nielsen, C. P. Radano, H. Sirringhaus, P. Smith and N. Stingelin-Stutzmann, Adv. Funct. Mater., 2007, 17, 2674–2679 CrossRef CAS.
- A. Goonesekera and S. Ducharme, J. Appl. Phys., 1999, 85, 6506–6514 CrossRef CAS PubMed.
- P. M. Borsenberger and H. Bässler, J. Chem. Phys., 1991, 95, 5327–5331 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Dependence of conductivity on film thickness and solution mixing time, TEM images for P3HT/PI samples and UV-Vis absorption spectra of films of pure P3HT, PS, PI and P3HT/PI blend. See DOI: 10.1039/c4ra12804d |
| This journal is © The Royal Society of Chemistry 2015 |