Ionic liquid–solvent mixtures as supercapacitor electrolytes for extreme temperature operation†
V.
Ruiz‡
,
T.
Huynh
,
S. R.
Sivakkumar§
and
A. G.
Pandolfo
*
CSIRO Division of Energy Technology, Box 312, Clayton South, Vic. 3169, Australia. E-mail: vruiz@cin2.es; tony.pandolfo@csiro.au; Fax: 9562 8919
First published on 13th April 2012
Abstract
Ionic liquid (IL)-based electrolytes containing molecular solvents were shown to be attractive for extreme temperature applications in electric double layer capacitors (EDLCs). In particular, the IL–butyronitrile (BuCN) mixture provides high capacitance (around 125 F g−1 at 500 mA g−1) independent of testing temperature, and superior performance at high current rates (reduced current dependence at high rates). Importantly, the IL–BuCN electrolyte can safely operate between −20 and + 80 °C, which overcomes the high temperature limitations of current commercial EDLCs. An additional advantage of IL–solvent mixtures is that the higher concentration of IL ions in the mixtures allows a greater specific capacitance (F g−1) to be achieved. The conductivity of the ionic liquid N-butyl-n-methylpyrrolidinium bis(trifluoromethane sulfonyl) imide (PYR14TFSI) could be increased from 2.48 mS cm−1 up to 45 mS cm−1 by mixing with an appropriate solvent. Importantly, these solvent mixtures also retain a wide electrochemical voltage window, in the range 4–6 V.
1. Introduction
The unique properties of room temperature ionic liquids (RTILs, organic salts liquid at room temperature) have attracted considerable interest particularly in applications where conventional liquids and/or solvents have operational or environmental limitations (e.g. extraction processes, metal deposition, catalysis, dissolution of natural biomaterials or energy management). In the area of energy management, RTILs have been studied for many years as alternative electrolytes,1,2 in various devices including: lithium-ion batteries,3 electrochemical actuators,4,5 dye-sensitized solar cells,6 and supercapacitors (SCs).7–9 The attraction of RTILs is due to their excellent physical properties which generally include: low volatility (with virtually no vapour pressure), high flame resistance, thermal and chemical stability together with a wide electrochemical potential window.1–11A well known challenge that the portable mobile energy storage industry faces is to develop safe, non-toxic and non-flammable devices that can operate in a wide temperature range, whilst still maintaining high energy density and good cycle life. In this respect, RTILs are excellent candidates as alterative, safe electrolytes in energy storage devices and in this study we explore their application in supercapacitors.
The most common commercial electrolytes used in supercapacitors are organic solvents containing a salt (typically an alkylammonium salt dissolved in acetonitrile, ACN). These mixtures, whilst having good conductivity and ion transport properties, also have high volatility, flammability and toxicity;7 which raise safety and environmental concerns. Moreover, the use of acetonitrile generally limits their applications above ∼70 °C due to the risk of cell rupture. It also has a very low flash point (10 °C) and emits toxic CN− and NOx as combustion products. The use of ILs therefore opens up the possibility of utilizing energy storage devices at higher temperature applications,1,12 such as military equipment, hand-held surgical tools for sterile surgical environments,13 subterranean probes and other power systems exposed to high temperature environments.
Currently, a major limitation of ILs for widespread applications is their relatively high viscosity14 as this property has a major impact on ionic mass transport. Whilst several ILs have shown very good performance above ambient temperatures,15 their viscosity increases substantially at sub-ambient temperatures resulting in a large increase in internal resistance and corresponding decrease in power output. Currently, commercially available RTILs do not have sufficient conductivity particularly at low temperatures, and so, several approaches have been undertaken in order to decrease their viscosity and improve their ionic conductivity.16–24 McEwen et al.25 improved the conductivity of ethylmethylimidazolium hexafluorophosphate (EMImPF6) by mixing with various organic solvents such as propylene carbonate (PC), ethylene carbonate (EC), dimethyl carbonate (DMC), diethyl carbonate (DEC) and ethyl methyl carbonate (EMC). They obtained a maximum conductivity of 27 mS cm−1 for a mixture of EMImPF6 with EC–DMC mixed solvent. In subsequent work by the same authors,6 another series of IL–solvent blends were investigated and the highest ionic conductivity was obtained for an acetone and EMImPF6 mixture (60 mS cm−1). Whilst this conductivity value is comparable with commercial acetonitrile electrolytes, the use of acetone will continue to be problematic at elevated temperatures. Jarosik et al.26 have also explored the effect of binary blends. They reported that binary IL mixtures (e.g. 1-ethyl-3-methyl imidazolium trifluoromethane sulfonate triflate (EMImTf) mixed with 1-ethyl-3-methylimidazolium bis(trifluoro methanesulfonylimide) (EMImNTf2)) sometimes displayed small synergistic improvements.27 That is, they display conductivity higher than the conductivity of each IL by itself, and higher than the conductivity expected from the weighted ratio of the two ILs. The conductivity increase was reportedly ∼1.3–1.6 times higher than expected: an effect the authors attributed to decreased ion association. However, this synergistic effect on conductivity was recorded at a single temperature (90 °C), and no indication of any improvements at lower temperatures was reported. Consistent with other reports, Jarosik et al.26 also observed that the greatest conductivity and viscosity improvements generally occurred with IL–solvent mixtures as the presence of the solvent has a greater disruptive effect on the ion pairing. A phosphonium protic ionic liquid was mixed with acetonitrile and used as the electrolyte for carbon-based supercapacitors28 showing improved performance; however, due to the thermal limitations of the solvent, they only reported results up to a temperature of 50 °C.
Despite the wide interest in IL mixtures, little data are available on the performance of IL–solvent mixtures in electrochemical capacitors, particularly both at low and elevated temperatures. The objective of this study is to improve the safety and operating temperature range of EDLCs by replacing the traditional acetonitrile–salt electrolyte mixture with a more benign electrolyte. Therefore, physical and electrochemical properties of the IL–solvent blends and their performance as electrolytes in EDLCs are evaluated at various operating temperatures in the range −20 °C to + 80 °C. The solvents selected in the present study are nitrile and carbonate-based solvents as these are already commonly used as electrolytes; however, there are still many members of these two classes of solvents that have not been well investigated as RTIL–solvent electrolyte mixtures in supercapacitors.
2. Experimental
2.1. Chemicals
N-Butyl-n-methylpyrrolidinium bis(trifluoromethane sulfonyl)imide (PYR14TFSI), supplied by Merck Chemicals (high purity grade, > 99%), was dried overnight at 60 °C under dynamic vacuum before use. Water content of the dried PYR14TFSI analysed with a Karl Fischer titrate indicated ≤30 ppm in the neat IL. Acetonitrile (ACN), butyronitrile (BuCN), benzonitrile (PhCN), benzyl cyanide (BnCN), propylene carbonate (PC), butylene carbonate (BC) and dimethylcarbonate (DMC) were supplied by Sigma–Aldrich, (anhydrous solvent grade) and were further dried under dynamic vacuum (where appropriate) before use. Preparation and testing of the IL–solvent mixtures were carried out inside a nitrogen glove box (O2 < 0.25 ppm and H2O < 0.1 ppm) to avoid any contamination.2.2. Physical properties
Ionic conductivity (σ, mS cm−1) of the neat IL and its corresponding mixtures was evaluated with a Mettler Toledo conductivity meter placed inside a glove box to ensure the inertness of the system. The RTIL was added in aliquots to the molecular solvent and stirred for 5 min until ionic conductivity remained steady for several minutes. Mixtures varying from 0 to 100 wt% in molecular solvent were evaluated. The concentration at which the mixture displays maximum ionic conductivity is denoted, Cσmax. Characterization of IL mixtures in EDLCs was carried out at Cσmax.Calorimetric measurements were made by using a differential scanning calorimeter (DSC) (TA Instruments, DSC 2910). An average of 5 mg of each sample was hermetically sealed in an aluminium pan inside a nitrogen glove box. Measurements were made from −150 °C to + 50 °C after cooling the sample with liquid nitrogen at a heating rate of 10 °C min−1, under a flow of N2 gas. The glass transition temperature, Tg, crystallisation temperature, Tc and melting temperature, Tm of the samples were obtained from the DSC thermogram.
The dynamic viscosity (η, cP) of the IL mixtures was determined at various temperatures (+20, +40, +60 and +80 °C) using an Anton Paar viscosity meter.
The electrochemical stability of the IL and its mixtures was examined at room temperature by cyclic voltammetry in a three electrode configuration, where glassy carbon was used as a working electrode and a platinum mesh as a counter electrode. The reference electrode was a silver wire immersed in a 10 mM silver trifluoromethanesulfonate (AgTf) solution in PYR14TFSI.
2.3. EDLC fabrication
Commercial activated carbon (AC) was obtained directly from the supplier (Maxsorb) and used as received. The AC electrodes were prepared by coating a mixture of AC (2.0 g), conductive carbon black (Printex L6, 0.4 g) and carboxymethyl cellulose salt binder (CMC, 0.2 g) dispersed in water, onto 30 μm thick aluminium foil using a grooved-rod applicator at a thickness of 36 μm. All the coated electrodes were roll-pressed and cut into 2.5 cm × 2.5 cm dimensions and vacuum dried overnight at 100 °C before cell assembly. Two electrode EDLC devices were assembled in laminated pouch-type cells. A 25 μm thick polypropylene separator was used as a separator. The neat ionic liquids and the IL mixtures (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 wt%) were used as electrolytes. For comparative purposes, performance of a EDLC using a widely used conventional organic electrolyte, 1 M TEABF4–ACN, was also evaluated. Electrochemical testing was performed at various temperatures (−20, +25, +50 and +80 °C) using a Solatron 1255B electrochemical analyser equipped with a 1470 multichannel battery test module. Galvanostatic cycling of the supercapacitors was carried out between 0 and 2.5 V at a maximum current density of 8 A g−1 (based on the weight of the active material on one of the electrodes). Specific capacitance values were obtained by applying the following equation: Ccapacitor (F) = I/(dV/dt) to the discharge curve (avoiding the ohmic drop).
1 wt%) were used as electrolytes. For comparative purposes, performance of a EDLC using a widely used conventional organic electrolyte, 1 M TEABF4–ACN, was also evaluated. Electrochemical testing was performed at various temperatures (−20, +25, +50 and +80 °C) using a Solatron 1255B electrochemical analyser equipped with a 1470 multichannel battery test module. Galvanostatic cycling of the supercapacitors was carried out between 0 and 2.5 V at a maximum current density of 8 A g−1 (based on the weight of the active material on one of the electrodes). Specific capacitance values were obtained by applying the following equation: Ccapacitor (F) = I/(dV/dt) to the discharge curve (avoiding the ohmic drop).
3. Results and discussion
For this study, n-butyl-n-methylpyrrolidinium bis(trifluoromethanesulfonyl) imide (PYR14TFSI) was chosen since this RTIL has a moderately low viscosity (100 cP) and is known to have a wide voltage stability window.29,30 ILs with the TFSI− anion show, in general, relatively low viscosity and high conductivity, due to the highly delocalized charge which reduces the interactions with neighbouring cations.31As discussed earlier, despite the favourable properties of this RTIL, its conductivity at ambient, and particularly at sub-ambient temperature, decreases markedly making it an ineffective or poor electrolyte at low temperatures. To improve the conductivity of the RTIL we need to increase the mobility of the ions by reducing their anion–cation attraction or “ion pairing” tendencies in their native state. To this end, solvents with a high dielectric constant have the ability to reduce the electric field surrounding an ion immersed in it. Polar aprotic solvents also tend to have large dipole moments (partial charge separation) and can solvate charged species via their induced dipole. Therefore, in selecting suitable solvents for this study, we focused on aprotic solvents, with high dielectric constants, low viscosities and acceptable melting and boiling points. In surveying suitable solvents we determined that many of the members of the nitrile and carbonate solvent families possessed suitable physical properties. Whilst this is not surprising since various carbonate and acetonitrile solvents are already commonly used as electrolytes, there are still many members of these two classes of solvents that have not been well investigated as RTIL–solvent electrolyte mixtures in supercapacitors.
3.1. Specific conductivity and dynamic viscosity
Table 1 summarizes the physical properties of the ionic liquid, PYR14TFSI, and the nitrile solvents evaluated in this study: acetonitrile (ACN), butyronitrile (buCN), benzonitrile (PhCN) and benzyl nitrile (BnCN). PYR14TFSI has a viscosity 100 cP and melting point of −18 °C. Its ionic conductivity is relatively poor (2.48 mS cm−1, Table 1) when compared to other conventional electrolytes (e.g. ∼60 mS cm−1 for 1 M tetraethylammonium tetrafluoroborate, TEABF4, dissolved in ACN). To improve the conductivity of the IL, a series of nitrile solvents were mixed with the neat IL in proportions ranging from 0 to 100 wt% and the ionic conductivity of each mixture was determined.| Neat IL | Molecular solvent | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| a Molecular weight (M), density (ρ), boiling point (BP), melting point (MP), ε dielectric constant, electrochemical window, EW (V vs. Ag|Ag+), concentration at which the mixture displays maximum ionic conductivity (Cσmax), measured ionic conductivity at 25°C (σ), measured dynamic viscosity at 20°C (η), activation energy (EA (Kcal mol−1)). N-butyl-n-methylpyrrolidinium bis(trifluorormethane sulfonyl) imide (PYR14 TFSI), acetonitrile (ACN), butyronitrile (BuCN), benzonitrile (PhCN), benzyl nitrile (BnCN), propylene carbonate (PC), butylene carbonate (BC) and dimethylcarbonate (DMC). | ||||||||||
| PYR14 TFSI | ACN | BuCN | PhCN | BnCN | DMC | PC | BC | |||
| M (g mol−1) | 422 | 41 | 69 | 103 | 117 | 90 | 103 | 116 | ||
| ρ (g cm−3) | 4.41 | 0.78 | 0.79 | 1.00 | 1.02 | 1.07 | 1.21 | 1.14 | ||
| BP(°C) | n/a | 82 | 117 | 191 | 233 | 90 | 240 | 250 | ||
| MP(°C) | −18 | −45 | −112 | −13 | −24 | 3 | −55 | −50 | ||
| ε | 38 | 20 | 26 | 18 | 3 | 64 | 58 | |||
| IL mixture | ||||||||||
| EW | 5.90 | 5.53 | 4.60 | 4.60 | 4.19 | 5.90 | 5.90 | 5.90 | ||
| Cσmax | — | 57 | 57 | 56 | 56 | 56 | 56 | 45 | ||
| σ (mS cm−1) | 2.48 | 45 | 20 | 10 | 8 | 15 | 12 | 9 | ||
| η (cP) | 100 | 1.6 | 2.3 | 4.3 | 6.7 | 3.5 | 6.2 | 7.8 | ||
| E A η | 7.06 | 1.84 | 2.51 | 3.43 | 3.80 | 3.02 | 3.88 | 4.34 | ||
| E A σ | 7.29 | 1.85 | 2.44 | 3.58 | 4.01 | — | 3.80 | 4.31 | ||
Fig. 1 shows the variation in the ionic conductivity of PYR14TFSI–nitrile solvent mixtures with solvent concentration (wt%). For all the mixtures studied, the ionic conductivity increased rapidly upon solvent addition, indicating an immediate decrease in the coulomb ion-pairing association between the cation and anion and their aggregates. With additional solvent, the conductivity values reached a maximum (Cσmax) at a solvent concentration in the vicinity ∼56–57 wt% (57 wt% for the mixture with ACN, 57 wt% for BuCN, 56 wt% for PhCN and 56 wt% for BnCN mixtures). These concentrations equate to solvent mole fractions, χML, of ∼0.93 and 0.89, for IL–ACN and IL–BuCN, respectively, and 0.84 for IL–PhCN and IL–BnCN mixtures. This trend of maximum conductivity has been previously reported by others 18,32,33 and is typical of salts dissolved in molecular solvent. Cσmax seems to show little dependence on the solvent utilized in this study, and whilst this may reflect the similarity of the solvents selected (e.g. high dielectric constants), a similar trend has been reported for solvents with differing properties.18,34,35 In the case of common salts, ionic conductivity can only be studied in a limited concentration range which is restricted by the solubility of the salt. However, unlike conventional salts, ILs are already in a liquid state at room temperature per se, and as they are soluble in organic solvents, and can form a single phase over a wider concentration range. The higher concentrations achievable with IL blends is an advantage of this type of system.
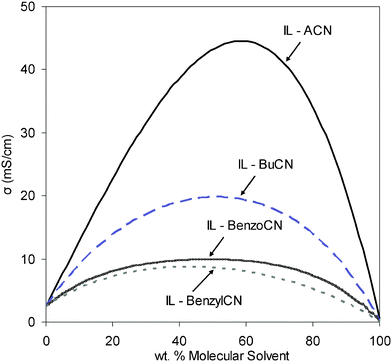 | ||
| Fig. 1 Ionic conductivity versus molecular solvent concentration for IL mixtures with several nitriles. Room temperature. | ||
Conductivity eventually decreases due to a dilution effect with the molecular solvent. Thus, the conductivity curve shows a smooth profile over the entire range of concentrations. At Cσmax, the improvement in conductivity observed for the IL–nitrile solvent mixtures is 3 to 16 times higher (depending on the solvent) than that of the neat IL. For the series of IL mixtures prepared, ionic conductivity values from 8–45 mS cm−1 were obtained (Table 1). Acetonitrile showed the most dramatic increase in conductivity attributed to its lower molecular weight, viscosity and density when compared with the other solvents studied (Table 1).
Accordingly, the viscosities of the nitrile mixtures were all visibly reduced by the addition of molecular solvents to the IL; from the initial 100 cP for the neat IL (at 20 °C), down to only 1.6–6.7 cP depending on the solvent (Table 1), (values were obtained at the point of maximum conductivity Cσmax). Results show a clear trend in the sense that the lower molecular weight of the solvent used in the mixture, the greater the reduction in viscosity.
The effect of the temperature on the conductivity and viscosity values for the neat IL and the solvent–IL mixtures was evaluated in the temperature range from +20 to +80 °C. Fig. 2a and 2b display the corresponding Arrhenius plot for viscosity and conductivity, respectively. In both cases the neat IL and the mixtures obey Arrhenius like behaviour (Ae−EA/RT). From the slope of such representations, the activation energies for the ionic conduction process in a viscous fluid, EAσ and EAη, were obtained (Table 1). After solvent addition, a decrease in activation energy can be observed indicating an increased mobility in the mixtures. The values of EA increased with the increase in molecular weight of the solvent used and a similar trend was found for both EAσ and EAη.
 | ||
| Fig. 2 (a) Viscosity and (b) conductivity Arrhenius plots for the neat IL and their mixtures with nitrile-derived solvents. (1) Neat IL, (2) IL–ACN, (3) IL–BuCN, (4) IL–PhCN, (5) IL–BnCN. | ||
In addition to the nitrile-based solvents, carbonate-based solvent mixtures (propylene carbonate (PC), butylene carbonate (BC) and dimethyl carbonate (DMC)) were also evaluated. The PYR14TFSI–carbonate solvent mixtures displayed similar conductivity trends with increasing solvent concentration to that observed for the nitrile mixtures (see ESI,† Fig. S1) reaching maximum conductivity at a concentration in the vicinity of Cσmax ∼ 56 wt% for the nitrile solvents with an exception; the mixture containing DMC presented a Cσmax ∼ 45 wt%). When using DMC, a lower amount of solvent is sufficient to reach the highest conductivity, although no clear explanation was found for this. Conductivity values in the range 9–15 mS cm−1 were recorded, which translates to a 3–6 fold increase when compared with the neat IL. There is a parallel reduction in the dynamic viscosity values, from 100 cP (neat IL) to 3.5–7.8 cP. Among all the carbonates used, the DMC–IL mixture showed the greatest improvement in conductivity (6 times higher than that of the neat IL). This is attributed to its lower molecular weight and viscosity; improving the dissociation of the IL ions and increasing their mobility. Arrhenius type behaviour was found for carbonate solvents (see ESI,† Fig. S.2a & 2b).
Combining all the information it can be concluded that the size of the molecular solvent used is the determining factor in controlling the ionic conductivity of the IL mixtures. Good correlation (r2 = 0.993) between the ionic conductivity and the reciprocal of the solvent molecular weight (which is often related to viscosity) was observed (Fig. 3), indicating that the solvent–solute interaction is the main driving force improving the ionic conductivity of the mixtures.25 Little correlation between the conductivity and the dielectric constant was found.
 | ||
| Fig. 3 Relationship between the ionic conductivity and molecular weight of the solvent used in the IL mixture. | ||
3.2. Electrochemical stability
While PYR14TFSI is known to have a wide and stable electrochemical window, an important consequence of mixing any solvent with PYR14TFSI can be a reduction in the electrochemical stability window of the mixture. The cyclic voltammogram for the neat IL and its mixtures is displayed in Fig. 4. The neat IL showed an impressive ∼6 V stability window, between the onset of PYR14+ cation reduction (−3.75 V vs. Ag|Ag+) and TFSI− anion oxidation (+2.15 V vs. Ag|Ag+), in agreement with literature reports.36 The nitrile-derived solvents reduced the electrochemical window of the neat IL to a greater extent than the carbonated solvents due to their lower electrochemical stability. Table 1 displays the electrochemical window values obtained for the mixtures, which vary between 4.19 and 5.90 V vs. Ag|Ag+. Fig. 4 also shows small additional currents during the forward and reverse scans, near the voltage limits of the IL mixtures, particularly for the carbonate mixtures. This additional small current is attributed to the presence of impurities in the IL–solvent mixtures. Electrolyte purity is an important issue with supercapacitor electrolytes and it is critical to remove all traces of impurities (down to very low ppm levels) from both the solvent and IL to ensure good long-term cycle life.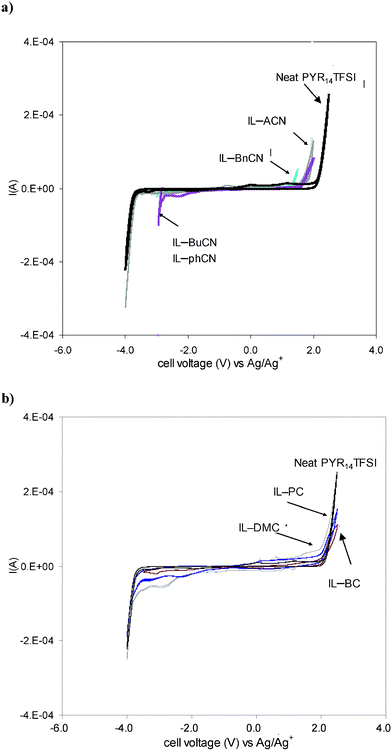 | ||
| Fig. 4 Cyclic voltammograms of neat IL and IL mixtures. (a) Nitrile based solvents and (b) carbonate based solvents. Three electrode configuration. WE: Glassy carbon, CE: Pt mesh, RE: Ag wire in 10 mM PYR14TFSI. | ||
3.3. Calorimetric data
The solid–liquid phase transitions of the neat IL and its mixtures were examined by differential scanning calorimetry (DSC) and the thermograms obtained are shown in Fig. 5. All the data were obtained from the first heating scan and the phase transitions were repeatable on a second heating scan. The physical properties (Tg, Tc and Tm) obtained from the DSC traces are summarised in Table 2. | ||
| Fig. 5 DSC traces of neat IL and its mixtures measured under flowing nitrogen. Heating rate: 10 °C min−1. | ||
| Transition | Neat IL | IL + ACN | IL + BuCN | IL + PhCN | IL + BnCN | IL + DMC | IL + PC | IL + BC |
|---|---|---|---|---|---|---|---|---|
| a Tg, glass transition temperature; Tc, crystallisation transition temperature; Tm, melting temperature. Tc and Tm values were taken from the respective transition peak maximum. Acetonitrile (ACN), butyronitrile (BuCN), benzonitrile (PhCN), benzyl nitrile (BnCN), propylene carbonate (PC), butylene carbonate (BC) and dimethylcarbonate (DMC). | ||||||||
| Tg (°C) | −87.67 | — | −97.05 | −108.99 | −104.15 | −95.06 | −107.28 | −106.71 |
| Tc (°C) | −52.13 | −71.46 | −66.91 | −67.48 | — | −48.14 | — | — |
| Tm (°C) | −15.73 | −62.36 | −22.56 | −21.7 | — | −41.32 | — | — |
The neat IL showed well-defined glass transition at low temperature (−87.67 °C) followed by crystallisation (−52.13 °C) and melting (−15.73 °C) on heating. There was also a small endothermic peak observed at −26.27 °C, which might represent essential thermal behaviour or the existence of contaminants. For the IL mixtures, two types of thermal behaviour are observed. The first type is represented by the IL mixtures prepared with PC, BC and BnCN showing only a small glass transition peak at low temperature, with no melting or crystallisation peaks observable. The disappearance of these peaks may suggest the existence of strong ion pairs in the solution.37 The second type of behaviour is characterized by the presence of a glass transition peak at a lower temperature (super cooled liquid behaviour) followed by an exothermic crystallisation peak (Tc) and a final melting (Tm, endothermic peak); which was observed for IL mixtures prepared with ACN, BuCN, PhCN and DMC. It is fortuitous that upon blending with solvents (except when DMC is used, which does not appreciably modify its value (−48 °C for IL–DMC and −52 °C for neat IL), all the phase transitions of the IL mixtures (Tg, Tc and Tm) are shifted towards more negative temperatures. This indicates that the liquid range of the IL mixtures is extended to more negative temperatures, a desirable property for supercapacitors operating at low temperatures. It should be noted that the crystallisation and melting transition temperatures of the IL mixtures do not simply follow the transition temperatures of either the neat IL or solvent (refer to Table 1 and 2), which indicates that there is an interaction between the solvent and the ion pairs in the IL mixtures. Also the nature of this interaction appears to vary with the type of solvent leading to the observed variation in crystallization behaviour pointed out earlier. That is, the crystallisation of ions are either shifted to more negative temperatures (as observed with ACN, BuCN, PhCN and DMC) or the crystallisation does not occur at all (as with PC, BC and BnCN) in the temperature range studied.
3.4. EDLC performance
The electrochemical performance of the neat IL and selected IL mixtures in EDLCs, at temperatures of −20, +25, +50 and +80 °C is shown in Fig. 6. Mixtures of IL–BuCN, IL–BC and IL–PC were selected for EDLC testing based on their combination of high electrochemical stability, ionic conductivity and high boiling point (as this will increase the working temperature range of the device). In addition, EDLCs employing conventional organic electrolyte used in commercial EDLCs (1 M TEABF4 in ACN) have also been included for comparative purposes. | ||
| Fig. 6 Specific capacitance values for the EDLCs at different temperatures: (a) −20 °C, (b) 25 °C, (c) 50 °C and (d) 80 °C. Electrolytes used: (1) neat IL, (2) IL–BuCN, (3) IL–BC, (4) IL–PC and (5) 1 M TEABF4–ACN. Voltage window: 2.5 V. Two electrode configuration. | ||
At −20 °C, EDLCs containing the neat IL electrolyte cannot be cycled (Fig. 6(a)), since it is below its melting point, −18 °C, Table 1. The capacitance values provided by IL–BC and IL–PC are fairly poor (below 50 F g−1 at low current densities) also showing a rapid decrease when the current density is increased. This is related to the low ion mobility at low temperature. These systems could only be cycled below 1–3 A g−1 due to the large IR drop observed during charge–discharge. In contrast, the IL–BuCN mixture showed high capacitance values 125 F g−1 (even higher than the conventional electrolyte) at the lowest current density (0.2 A g−1) and ∼70 F g−1 at the highest (5 A g−1). Both 1 M TEABF4–ACN and IL–BuCN electrolytes show a marked decrease in capacitance values when the current density is increased. This is related to the higher resistance of the system (lower ionic conductivity) at −20 °C.
As the testing temperature is increased to room temperature (Fig. 6(b)) the capacitance values increase and showed improved performance at higher current densities. The neat IL still displayed relatively poor performance at +25 °C. On the contrary, IL–PC and IL–BC showed great improvement while the 1 M TEABF4–ACN and IL–BuCN electrolytes continued to display the best performance of all the electrolytes tested. It is worth noting that the capacitance provided by the IL–BuCN mixture continues to be higher than the commercial electrolyte (from 8 to 18% higher at low to high current densities, respectively). As the mixture consists of around 55 wt% of IL in solution, its molarity is higher that the 1 M concentration used in the TEABF4–ACN electrolyte (around 1.18 M). Hence, more ions are available for the double layer formation process during charge storage and hence a greater capacitance can be expected in these IL-based electrolytes.
The neat IL required a temperature increase to +50 °C (Fig. 6(c)) in order to provide comparable capacitance values to other electrolytes at low current densities; however, its low conductivity still restricts its performance at higher current density. At +50 °C, the C (F g−1) vs. I (A g−1) profiles for TEABF4–ACN and the IL–BC and IL–PC mixtures are very similar. Once again, the IL–BuCN electrolyte showed superior performance at this temperature.
At 80 °C the cell containing the neat IL electrolyte recorded the highest capacitance values (in the range of current densities from 1–4 A g−1) thereby highlighting the advantages of using neat ionic liquids operating at relatively high temperatures. At this temperature the neat IL is sufficiently fluid to attain good mobility (conductivity) and the absence of a solvent enables a high ion concentration, and hence, a greater double layer capacitance is attained. The IL–solvent mixtures again displayed good capacitance vs. current density performance. As ACN boils at 82 °C, the TEABF4–ACN system was not tested at +80 °C (as it typically causes cell rupture/failure) stressing the current limitation of using low boiling point solvents in supercapacitors.
4. Conclusions
The conductivity of the ionic liquid, N-butyl-n-methylpyrrolidinium bis(trifluoromethane sulfonyl) imide (PYR14TFSI) was increased from 2.48 mS cm−1 to 8–45 mS cm−1 by mixing with an appropriate nitrile or carbonate-based solvent. The improvement in ionic conductivity is achieved by reducing the ion pairing association of the neat ionic liquid, via solvation, thereby decreasing the viscosity of the system and increasing ion mobility. Importantly, these solvent mixtures also retained a wide electrochemical voltage window of between 4 and 6 V, depending on the solvent.The IL–solvent mixtures were shown to be excellent electrolytes for EDLCs and could operate over a wide range of temperatures (−20 to +80 °C). In particular, the IL–butyronitrile (BuCN) mixture provided higher capacitance (around 125 F g−1 at 500 mA g−1; independent of the testing temperature) and superior performance at high current rates, than both the commercially employed electrolyte in supercapacitors (1 M TEABF4–acetonitrile) and the neat IL. Importantly, the IL–BuCN electrolyte can safely operate between −20 and at +80 °C. Potentially, electric double layer capacitors (EDLCs) with IL–BuCN electrolyte could be operated at even at higher temperatures as the boiling point of BuCN is +117 °C. This would overcome the high temperature limitations of current commercial EDLCs that are limited by the low boiling point of acetonitrile. An additional advantage of the IL–solvent mixtures is that the higher concentration of IL ions in the mixtures allows a greater capacitance to be achieved.
From the results obtained, PYR14TFSI-based systems containing molecular solvents can be regarded as promising alternative electrolytes for EDLC applications where wide temperature stability ranges are required. Further work is underway to define the upper temperature limits of these systems and their long term (cycling) stability.
Acknowledgements
This work was funded through the CSIRO Energy Transformed Flagship. Authors acknowledge Dr Rob Rees for providing ionic liquid PYR14TFSI image generated with GaussView software.References
- Electrochemical Aspects of Ionic Liquids, ed. Hiroyuki Ohno, Chapter 1: Importance and Possibility of Ionic Liquids, John Wiler & Sons, Inc., Publication, 2005 Search PubMed.
- J. F. Brennecke and E. J. Maginn, AIChE J., 2001, 47, 2384 CrossRef CAS.
- H. Sakaebe and H. Matsumoto, Electrochem. Commun., 2003, 5, 594 CrossRef CAS.
- M. D. Bennnett, D. Leo and J. Sens, Sens. Actuators, A, 2004, 115, 79 CrossRef.
- J. Ding, D. Zhou, G. Spinks, G. Wallace, S. Forsyth, M. Forsyth and D. MacFarlane, Chem. Mater., 2003, 15, 2392 CrossRef CAS.
- A. B. McEwen, H. L. Ngo, K. LeCompte and J. L. Goldman, J. Electrochem. Soc., 1999, 146(5), 1687 CrossRef CAS.
- M. Mastragostino and F. Soavi, Capacitors. Electrochemical Capacitors: Ionic Liquid Electrolytes, Encyclopedia of Electrochemical Power Sources Search PubMed , J. Garche, C. Dyer, P. Moseley, Z. Ogumi, D. Rand, B. Scrosati. Elsevier, Amsterdam, 2009, p. 649.
- K. Yuyama, G. Masuda, H. Yoshida and T. Sato, J. Power Sources, 2006, 162, 1401 CrossRef CAS.
- T. Sato, G. Masuda and K. Takagi, Electrochim. Acta, 2004, 49, 3606 CrossRef.
- M. Galinski, A. Lewandowski and I. Stepniak, Electrochim. Acta, 2006, 51(26), 5567 CrossRef CAS.
- M. Armand, F. Endres, D. R. MacFarlane, H. Ohno and B. Scrosatti, Nat. Mater., 2009, 8, 621 CrossRef CAS.
- T. Abdallah, D. Lemordant and B. Claude-Montigny, J. Power Sources, 2012, 201, 353 CrossRef CAS.
- R. S. Tichy, Battery Power Products & Technology Magazine, 2007, 11, 3 Search PubMed.
- P. Hapiot and C. Lagrost, Chem. Rev., 2008, 108(7), 2238 CrossRef CAS.
- A. Balducci, F. Soavi and M. Mastragostino, Appl. Phys., 2006, A82, 627 Search PubMed.
- T. Nishida, Y. Tashiro and M. Yamamoto, J. Fluorine Chem., 2003, 120, 135 CrossRef CAS.
- G. B. Appetecchi, M. Montanino, A. Balducci, S. F. Lux, M. Winter and S. Passerini, J. Power Sources, 2009, 192, 599 CrossRef CAS.
- Y. François, K. Zhang, A. Varenne and P. Gareil, Anal. Chim. Acta, 2006, 562, 164 CrossRef.
- T.-Y. Wu, H.-C. Wang, S.-G. Su, S.-T. Gung, M.-W. Lin and C.-b. Lin, J. Taiwan Inst. Chem. Eng., 2010, 41, 315 CrossRef CAS.
- A. Zhu, J. Wang, L. Han and M. Fan, Chem. Eng. J., 2009, 147, 27 CrossRef CAS.
- R.-S. Kühnel, N. Böckenfeld, S. Passerini, M. Winter and A. Balducci, Electrochim. Acta, 2011, 56, 4092 CrossRef.
- B. Singh Lalia, N. Yoshimoto, M. Egashira and M. Morita, J. Power Sources, 2010, 195(21), 7426 CrossRef.
- M. Diaw, A. Chagnes, B. Carré, P. Willmann and D. Lemordant, J. Power Sources, 2005, 146(1–2), 682 CrossRef CAS.
- A. Krause and A. Balducci, Electrochem. Commun., 2011, 13, 814 CrossRef CAS.
- A. B. McEwen, S. F. McDevitt and V. R. Koch, J. Electrochem. Soc., 1997, 144(4), L84 CrossRef CAS.
- A. Jaroski, R. Krajewski, A. Lewandowski and P. Radzimski, J. Mol. Liq., 2006, 123, 43 CrossRef.
- H. Every, A. G. Bishop, M. Forsyth and D. R. MacFarlane, Electrochim. Acta, 2000, 45, 1271 CrossRef.
- L. Timperman, H. Galiano, D. Lemordant and M. Anouti, Electrochem. Commun., 2011, 13, 1112 CrossRef CAS.
- A. Lewandowski and A. Swiderska-Mocek, J. Power Sources, 2009, 194, 601 CrossRef CAS.
- A. Balducci, M. Schmuck, W. Kern, B. Rupp, S. Passerini and M. Winter, ECS Trans., 2008, 11, 109 CrossRef CAS.
- I. Rey, P. Johansson, J. Lindgren, J.-C. Lassègues, J. Grondin and L. Servant, J. Phys. Chem. A, 1998, 102, 3249 CrossRef CAS.
- P. Radzimski, MSc Thesis, 2004, Poznan University of Technology Search PubMed.
- A. Noda, Md. Abu Bin Hasan Susan, K. Kudo, S. Mitsushima, K. Haymmizu and M. Watanabe, J. Phys. Chem., 2003, B107, 4024 Search PubMed.
- P. Radzimski, MSc Thesis, 2004, Poznan University of Technology Search PubMed.
- A. Noda, Md. Abu Bin Hasan Susan, K. Kudo, S. Mitsushima, K. Haymmizu and M. Watanabe, J. Phys. Chem., 2003, B107, 4024 Search PubMed.
- S. Zhang, N. Sun, X. He, X. Lu and X. Zhang, J. Phys. Chem., 2006, 35(4), 1475 CAS.
- M. Egashira, S. Okada, J.-I. Yamaki, N. Yoshimoto and M. Morita, Electrochim. Acta, 2005, 50, 3708 CrossRef CAS.
Footnotes |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra20177a/ |
| ‡ Present address: CIN2-CSIC. Campus UAB. Building Q, 08193 Bellaterra, Cataluña, Spain |
| § Present address: School of Chemical & Biotechnology, SASTRA University, Thanjavur - 613 401, India |
| This journal is © The Royal Society of Chemistry 2012 |
