MOF nanozymes: active sites and sensing applications
Ziyan
Zhang†
a,
Yujie
Li†
ab,
Zhishuang
Yuan
a,
Lingxia
Wu
a,
Jiping
Ma
a,
Weiqiang
Tan
a,
Yingjie
Sun
a,
Guangyao
Zhang
 *b and
Huining
Chai
*b and
Huining
Chai
 *a
*a
aSchool of Environmental and Municipal Engineering, Qingdao University of Technology, Qingdao 266520, China. E-mail: chaihuining@qut.edu.cn
bIntelligent Wearable Engineering Research Center of Qingdao, Research Center for Intelligent and Wearable Technology, College of Textiles and Clothing, State Key Laboratory of Bio-Fibers and Eco-Textiles, Qingdao University, Qingdao 266071, China. E-mail: gyzhang@qdu.edu.cn
First published on 27th November 2024
Abstract
Metal–organic frameworks (MOFs) are porous organic–inorganic coordination materials with numerous active sites, enabling them to mimic the properties of natural enzymes and making them highly promising for sensing applications. This review provides a detailed overview of recent advancements in leveraging MOFs for the design of catalytic active sites in nanozymes. MOFs utilize metal ions and organic ligands as active centers for biomimetic catalysis, while their porous frameworks efficiently bind and stabilize multiple guest active units. Furthermore, MOFs can undergo chemical transformations to produce derivatives such as porous carbon materials and nanostructured metal compounds, enhancing their catalytic performance and broadening their applications as nanozymes. This review also explores the progress of MOF-based nanozymes across various catalytic modes in analytical sensing, highlighting their ability to significantly improve detection sensitivity, selectivity, and range. Additionally, the critical role of diverse active sites in sensing processes is emphasized, with attention to the design and synthesis strategies required to optimize the performance of MOF nanozymes. Finally, the review discusses future prospects for the development of MOF nanozymes and outlines key challenges that must be addressed to advance this field.
1. Introduction
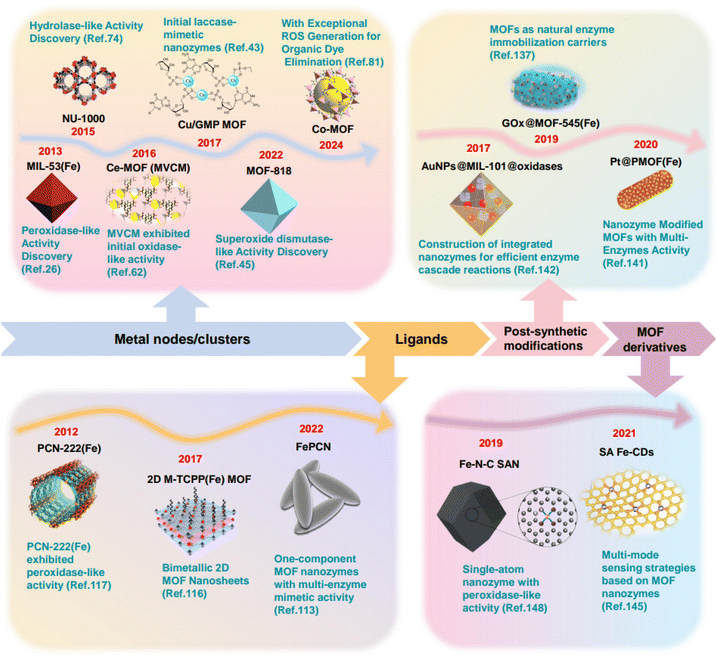
Natural enzymes, as highly efficient and specific biocatalysts derived from proteins, demonstrate exceptional substrate selectivity and catalytic activity under mild conditions. In several areas, they play a crucial role, including life sciences, biomedicine, biochemical analysis, agricultural science, food processing, and environmental remediation. However, the high costs of preparation, limited stability, and time-intensive production processes of natural enzymes significantly constrain their broader application in pharmaceuticals and industry. To address these limitations, extensive research has been undertaken to develop artificial enzymes that have the capacity to mimic the catalytic activities and specificities of natural enzymes.In the context of the integration of biotechnology and nanotechnology, nanozymes, a novel class of nanomaterials, have emerged and were recognized as one of the top ten emerging chemical innovations in 2022 by the international union of pure and applied chemistry (IUPAC). Compared to natural enzymes, nanozymes possess advantages such as high efficiency, facile preparation, and low cost, highlighting their significant potential for diverse application.1–3 Nanozymes reported in the literature can be broadly categorized into four main types: carbon-based nanozymes (e.g., graphene, carbon nanotubes), metal-based nanozymes (e.g., gold, silver, platinum nanoparticles), metal oxide-based nanozymes (e.g., CeO2, Fe2O3, MnO2), and other emerging nanozymes, including metal–organic frameworks (MOFs)4–7 and covalent organic frameworks (COFs). Among these, the creation of MOF nanozymes, which are constructed from metal ions/clusters and organic ligands, has received considerable attention (Fig. 1).8–11 Firstly, the active centres of certain natural enzymes can be mimicked by the metal nodes/clusters and organic ligands in MOFs, thus bestowing MOFs with intrinsic enzymatic activity.12 Secondly, MOFs can be functionalized with various groups, enabling the grafting of required active sites onto metal ions/clusters or organic ligands. Additionally, the inherent porosity of MOFs facilitates the encapsulation of natural enzymes and the construction of multi-enzyme cascade systems.13 Meanwhile, MOF derivatives can be obtained through processes such as pyrolysis and etching, which retain portions of the framework structure and active sites of the MOF precursors, while still exhibiting excellent catalytic activity.14
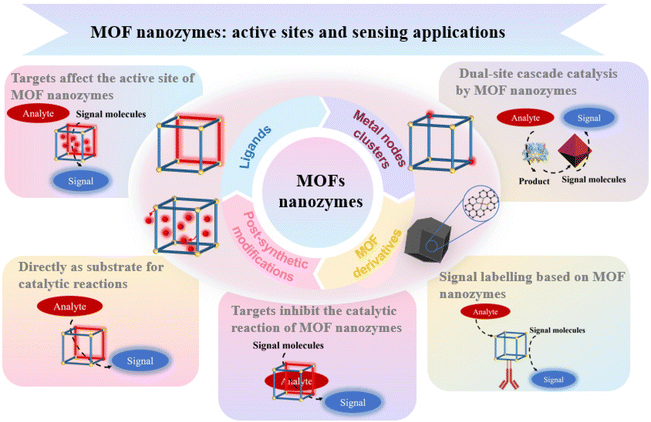
To date, research on MOF-based nanozymes has made significant strides, and the number of related reviews is steadily increasing. Existing reviews mainly categorize MOFs according to their structure, preparation methods, or types of enzyme activities.15 However, discussions on the relationship between enzyme activity and MOFs structure, as well as how to effectively integrate enzyme active sites with the MOFs framework, are still relatively scarce. Therefore, this review combines enzyme-like activity with the structural characteristics of MOFs, providing a detailed summary of the origins of enzyme-mimicking activity through a systematic classification of catalytic active sites. Subsequently, based on the sensing mechanisms of catalytic modes, we summarize the research progress of MOF nanozymes in analytical sensing, including direct catalytic substrate sensing modes, indirect modulation of catalytic activity sensing modes, cascade catalytic sensing modes, inhibition catalytic sensing modes, and signal tag-based sensing modes. These modes demonstrate the versatility and high selectivity of MOF nanozymes in the sensing field. Finally, this review summarizes the development prospects and key challenges that need to be addressed for MOF nanozymes (Fig. 2).
2. Active sites of MOF nanozymes
MOF nanozymes, characterized by their porous structures and outstanding catalytic activity,16 have found increasing applications in the field of sensing. By integrating their active sites into sensors, precise recognition and rapid responses to analyte molecules can be achieved. The ordered alignment of metal nodes and organic ligands in MOFs provides plentiful and easily accessible active sites. Their high specific surface area greatly facilitates post-synthetic modifications, enabling the addition of more active sites. Moreover, the porous structure of MOFs can accommodate various additional active substances (e.g., inorganic nanoparticles, enzymes), further enhancing their sensing performance. MOFs can also act as precursor templates for synthesizing various types of MOF-derived nanozymes. These structurally diverse MOF-derived nanozymes are characterized by uniform active sites. Integrating the active sites of MOF nanozymes into sensors not only improves their sensitivity and selectivity but also enhances their stability and reliability in complex environments.2.1 Metal nodes/clusters as active sites
The enzyme-like catalytic activities of many MOF-based nanozymes are intimately linked to the active centers of their metal nodes or clusters. In these MOFs, inorganic metal ions are linked to labile ligands (e.g. solvent molecules) that can be removed during activation, thus preserving the framework structure and generating coordinatively unsaturated sites (CUSs) within the metal nodes. As typical Lewis acid centres, CUSs can accept electrons from substrates, facilitating their transformation into products, thereby exhibiting enzyme-like activity. MOFs containing metal nodes such as Fe, Cu, Co, Ni, or Ce, Zr exhibit enzyme-like catalytic activity due to the presence of these CUSs. Recent studies have reported numerous MOFs exhibiting various enzyme-like activities, including but not limited to peroxidase (POD), oxidase (OXD), superoxide dismutase (SOD), catalase (CAT), and hydrolase activities. Incorporating these MOF nanozymes with distinct enzyme-like activities into sensors can greatly improve their performance. The presence of CUSs enables sensors to interact more efficiently with analyte molecules, thereby improving their sensitivity and selectivity. | ||
| Fig. 3 (A) Schematic illustration of the peroxidase-like activity of Fe-MIL-88NH2 in catalysis of the TMB–H2O2 system. (Reproduced from ref. 21.) (B) OXD-like activity of MIL-53(Fe). (Reproduced from ref. 31.) (C) (a) Catalytic TMB oxidation activities of MIL-53(Fe)-X MOFs plotted as a function of Hammett σm values of the linker substituent X. Values of kX were calculated from initial kinetic observations. The gray line is shown as a visual guide only. X = NH2, CH3, H, OH, F, Cl, Br, and NO2. (b) Proposed catalytic cycle for the oxidation of TMB to TMB+ with MIL-53(Fe)-X as oxidase mimics (reproduced from ref. 32). | ||
Oxidases are a class of copper- or iron-containing protein enzymes capable of activating molecular oxygen and directly facilitating the oxidation of specific substrates. Their catalytic activity primarily stems from the active centers within the protein structure. However, MOFs composed of metal ions and organic ligands face challenges in mimicking these protein active centers, and Fe-based MOFs exhibiting OXD-like activity30 are relatively rare. Jiang et al.31 were the first to demonstrate that the synthesized MIL-53(Fe) exhibits intrinsic OXD-like catalytic activity, enabling it to efficiently catalyze the oxidation of TMB using molecular oxygen as the terminal electron acceptor. This catalytic mechanism is attributed to the spontaneous redox cycling between Fe2+ and Fe3+ ions within the material (Fig. 3B). A colorimetric sensor for the detection of biothiols was developed based on this discovery. Compared to nanozymes, proteases have clearly defined active sites and neighbouring micro-environments or residues, such as primary and secondary coordination spheres, which allow for rational design and study of their structure–activity relationships. Inspired by protein engineering, the metal–ligand coordination in MOFs can be rationally adjusted through ligand engineering strategies to fine-tune their enzyme-like activity. Wei et al.32 selected MIL-53(Fe) as a model and designed a series of MOF materials with similar coordination structures by varying the linker substituent X in MIL-53(Fe)-X (where X = NH2, CH3, H, OH, F, Cl, Br, and NO2). They explored the relationship between the structure and oxidase performance (Fig. 3C(a)). By combining experimental results with DFT calculations, they demonstrated that MIL-53(Fe) exhibits a linear free energy relationship (LFER) between the Hammett structure and catalytic performance. Specifically, when the ligand structure in the material is electron-deficient, the Hammett σm value increases, resulting in enhanced enzymatic catalytic performance (Fig. 3C(b)). In 2015, Wu et al.33 were the first to report a multienzyme-like system based on MOFs. The multienzyme-like activity can endow MOF nanozymes with excellent anti-interference capabilities, thereby improving sensor stability. Currently, Fe-based MOFs exhibiting multienzyme-like activity are primarily realized in the form of multi-component nanozymes, while developing single-component nanozymes with multienzyme-like activity represents a new direction for future research.27
Laccase is a multicopper oxidase with a catalytic core containing four copper ions, capable of efficiently catalyzing the oxidation of substrates such as lignin and phenols through a four-electron transfer process.37 Inspired by the multi-metallic complex structure of laccase, researchers have developed Cu-based MOF nanozymes38–42 with laccase-like activity. Liang et al.43 demonstrated a copper-based laccase mimic coordinated with guanosine monophosphate (GMP). This formed an amorphous MOFs material with excellent laccase-like activity, capable of converting various phenolic substrates. Comparative studies indicated that this activity is attributed to the specific coordination between the copper ions and guanosine (Fig. 4A). Compared to protein-based laccase, the Cu/GMP nanozyme showed a higher Vmax and a similar Km at the same mass concentration. In comparison to crystalline metal–organic frameworks, the higher heterogeneity and defect structures of amorphous MOFs result in higher catalytic activity, which contribute to enhanced sensor performance. Wang et al.44 prepared an amorphous MOF nanozymes (CA-Cu), which exhibited higher laccase-like and catechol oxidase-like activities compared to laccase and the previously reported MOF-818. This exceptional efficiency is achieved by the synergistic effects of its internal dinuclear Cu(II) and dinuclear Cu(I) sites, which efficiently bind substrate molecules and O2, respectively, thereby enabling effective catalysis and detection of phenolic compounds (Fig. 4B). Additionally, the coordination of nitrogen with copper serves as the active site of the CA-Cu nanozyme, while the coordination of hydroxyl groups with copper merely enhances structural stability.
 | ||
| Fig. 4 (A) Active site of Cu/GMP. (Reproduced from ref. 43.) (B) Schematic illustration of the synthesis of the CA-Cu nanozyme with laccase- and catecholase-like activity by mimicking their catalytic center. (Reproduced from ref. 44.) (C) (a) Structure of MOF-818 and catechol oxidase. (b) The scheme of detecting H2O2. (Reproduced from ref. 45.) (D) (a) Schematic illustration of the possible mechanism of peroxidase-like activity of MOF-818. (b) UV-vis absorption spectra of TMB + H2O2 + MOF-818 (a), TMB + H2O2 + UiO-66 (b), TMB + H2O2 (c), TMB + MOF-818 (d). (c) ESR spectra of H2O2 + DMPO and MOF-818 + H2O2 + DMPO. (Reproduced from ref. 46.) | ||
Compared with natural enzymes, MOF nanozymes are defective in selectivity; natural enzymes have extremely high substrate selectivity because they have complex three-dimensional structures that can form precise spatial matches with specific substrate molecules. In contrast, although MOFs nanozymes can mimic certain catalytic properties of natural enzymes by designing pore structures and active sites, their structural complexity and specificity are usually inferior to those of natural enzymes. Li et al.45 prepared MOF-818 (Fig. 4C(a)), which exhibits catechol oxidase-like activity but lacks POD-like activity. MOF-818 can catalyze the oxidation of catechol to the corresponding quinone through its trinuclear copper center in the presence of oxygen, simultaneously producing H2O2 instead of H2O as an intermediate (Fig. 4C(b)). By imitating the active centre of natural catechol oxidase, MOF-818 has superior catalytic capacity and selectivity compared to existing oxidase nanozymes. The same MOF nanozymes can exhibit different enzyme-like activities under varying conditions. Our research group46 successfully synthesized MOF-818 (Fig. 4D(a)), which exhibited excellent POD-like activity (Fig. 4D(b)) at 37 °C and under acidic conditions, efficiently catalyzing H2O2 to produce ˙OH and oxidize the substrate TMB (Fig. 4D(c)). The root of its catalytic activity lies in the trinuclear copper center of MOF-818. Based on this, it is possible to detect H2O2 and H2S released by living cells. Hydrolases include enzymes capable of catalyzing various hydrolysis reactions, such as carbonic anhydrase, lipase, and protease. Currently, the hydrolase-mimicking MOFs can be categorized into three types: organophosphorus hydrolase, protease, and esterase, with Cu-based MOF nanozymes primarily exhibiting protease activity. The research by Li et al.47 showed that the HKUST-1 material exhibits inherent mimic enzyme activity similar to natural trypsin, particularly in catalyzing bovine serum albumin (BSA) and casein, with a catalytic mechanism highly consistent with that of natural trypsin. The Km of Cu-MOFs is approximately 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times lower than that of free trypsin, which indicates a much higher albumin affinity for the surface of Cu-MOFs.
000 times lower than that of free trypsin, which indicates a much higher albumin affinity for the surface of Cu-MOFs.
Additionally, researchers have developed Cu-based MOFs with POD-like activity.48–51 In these, Cu2+ can effectively catalyze the decomposition of H2O2, generating highly active ˙OH radicals. Tan et al.52 first demonstrated that HKUST-1 exhibits POD-like activity, efficiently catalyzing the oxidation of thiamine (TH) by H2O2. Based on its POD-like activity, a sensitive fluorescent detection method for TH was developed, leading to broader applications in bioanalysis. The catalyst activity of MOF nanozymes can be modified by tailoring different ligands to improve sensor performance. In addition to optimizing catalytic activity by adjusting MOF components, controlling the structural dimensions of MOFs can further enhance their applications in sensing. Shi et al.53 demonstrated that Cu-based two-dimensional MOF nanosheets (Cu(bpy)2(OTf)2) exhibit POD-like activity, with activity superior to that of three-dimensional Cu-MOFs. The coordination of pyridine to copper in these nanosheets is stable under alkaline conditions, with bpy ligands stably connecting with Cu(II) to form a two-dimensional network, while OTf acts as a spacer ligand during exfoliation. This allows for high-sensitivity fluorescence detection of H2O2 and glucose. Most reported Cu-based MOFs only exhibit single nanozyme activity, making it crucial to further develop Cu MOFs with multiple enzyme-like activities. Ying et al.54 synthesized hexagonal prism-shaped Cu FMA by using CuCl2 as the source of copper and fumaric acid (FMA) as the organic ligand. This material exhibits laccase-like activity under alcaline conditions and POD-like activity under acidic conditions. The specificity of Cu FMA at different pH values may be due to the weak reducing property of FMA, which introduces the presence of Cu+ active centers. At pH = 8, the Cu+ active center favors the dissociation of hydroxyl bonds in phenolic compounds. Conversely, at pH = 4, the dissociation of H–O is weakened, promoting the cleavage of the O–O bond in H2O2. Based on its dual enzyme-like activities, the Cu FMA sensor can effectively detect glucose and adrenaline in human serum.
Superoxide dismutase (SOD) is one of the body's primary antioxidants, capable of catalysing the reduction of superoxide radicals (O2˙−) to H2O2 and O2, thereby protecting cells and treating diseases associated with oxidative stress.57 Liu et al.58 designed two monovalent Ce-MOFs with SOD-like activity. The core of the SOD-like catalytic mechanism in these Ce-MOFs lies in the cyclic conversion between Ce ions (Ce3+ and Ce4+). Additionally, the changes in these oxidation states also endow Ce-MOFs with excellent OXD-like activity.59–61 Xiong et al.62 were the first to demonstrate that the synthesized Ce-based MOFs (MVCM) possess intrinsic OXD-like catalytic activity. Based on the outstanding catalysis capability of MVCM, a colorimetric method was developed for detecting biothiols in serum, showing great potential for applications in analytical sensing. Catalase (CAT) facilitates the decomposition of H2O2 into molecular oxygen and water, thereby removing hydrogen peroxide from the body. Abdelhamid et al.63 successfully synthesized a novel Ce-MOF with catalase-like activity using the organic linker 4,4′,4′-trihydroxymethylbenzoic acid (H3NTB). This MOFs is formed by the coordination of cerium clusters with the trihydroxyl linker H3NTB, exhibiting excellent thermal stability and unique optical properties, making it highly promising for applications in biosensing. Utilizing the CAT-like activity of Ce-MOF, successful sensing of H2O2 and Fe3+ was achieved.
Additionally, the Ce clusters in MOFs can effectively hydrolyze phosphate ester bonds, exhibiting alkaline phosphatase (ALP)-like activity.64 In Ce MOFs, the properties of the ligands are crucial to their catalytic performance. These ligands not only exhibit high electron affinity but also significant π–π stacking interactions, which have a notable impact on facilitating the redox reactions of Ce. The interactions between Ce and the ligands can effectively regulate the transition between its oxidation states, thereby enhancing the OXD-like catalytic activity of Ce MOFs and improving sensor performance. Olloqui-Sergio et al.65 synthesized UiO-66(Ce), which contains Ce4+/Ce3+ as an intrinsic redox center. The terephthalic acid (TA) ligand in the MOFs has highly delocalized π-electrons, which will facilitate the reversible Ce4+/Ce3+ redox conversion, thereby enhancing the enzyme-like catalytic activity of the MOFs. Similar to other metal-based MOF nanozymes, some Ce-based MOF nanozymes also exhibit multi-enzyme activity. Luo et al.66 reported a mixed-valence Ce-BPyDC, which exhibits dual OXD-like and POD-like activities (Fig. 5A). Ce-BPyDC retains the coexistence of Ce3+ and Ce4+ within the MOFs structure, and due to its redox-active Ce3+/Ce4+ cycle, Ce-BPyDC exhibits excellent catalytic activity. Based on this, a colorimetric sensor platform for AA has been established, expanding the application of this mixed-valence MOF nanozymes in the field of sensing.
 | ||
| Fig. 5 (A) Illustration of colorimetric detection of AA by using Ce-BPyDC as a catalyst. (Reproduced from ref. 66.) (B) Schematic illustration showing the biomimetic-strategy for construction of multivalent Ce-MOFs as novel laccase nanozymes for environmental remediation. (Reproduced from ref. 71.) (C) Molecular representations of the NU-1000 node and linker (left), MOFs topology (two views, centre), and the dehydration of the NU-1000 node (right). Colour code: Zr (blue); O (red); C (black); H (white). (Reproduced from ref. 74.) (D) Schematic illustration showing the biomimetic-strategy for construction of multivalent Ce-MOFs as novel laccase nanozymes for environmental remediation. (Reproduced from ref. 79.) (E) Schematic of GSH detection using the D-ZIF-67 nanosheet as a nanozyme. (Reproduced from ref. 86.) (F) Schematic Illustration properties of ZIF-L-Co-10 mg Cys. (Reproduced from ref. 87.) (G) (a) Schematic illustration of bi-metallic MOF-919 (Fe–Cu) mimicking bifunctional oxidase–peroxidase catalytic activity. (b) Oxidase and peroxidase activity monitoring by UV-vis absorption spectroscopy. (Reproduced from ref. 103.) (H) Illustration of fluorescence and colorimetric dual-mode detection of vanillin based on MIL-101 (Co,Fe)@MIP. (Reproduced from ref. 104.) | ||
The currently reported laccase mimics are mainly focused on materials that contain copper67–70 with copper as the catalytic active center, while non-copper-based laccase mimics have not yet received sufficient attention. Drawing on the mechanism of multiple copper active centers and the electron transfer between Cu2+/Cu+ in the redox process found in natural laccases, Ce-MOFs rich in Ce(III)/Ce(IV) redox pairs are considered potential candidates for laccase mimics due to their inherent redox properties. Laccase-like activity was observed in a series of multivalent Ce-MOFs designed by Liang et al.71 where the intrinsic redox properties of Ce4+/Ce3+ can mimic the Cu2+/Cu+ redox electron transfer pathway of natural laccases. Compared to natural laccases, Ce-UiO-66 and Ce-MOF-808 show higher initial rates (V0) and lower Km values (Fig. 5B).
MOFs containing Zr nodes can mimic the activity of organophosphorus hydrolases,72–74 rapidly hydrolyzing phosphoester bonds, and thus have potential applications in degrading organophosphorus warfare agents. This hydrolase-mimicking activity is primarily attributed to the specific interactions between Zr–O clusters and molecules. The Zr-containing MOFs (NU-1000) prepared by Mondloch et al.74 can effectively promote the hydrolysis of phosphoester bonds (Fig. 5C). NU-1000 uses Zr6 as the metal node, with 1,3,6,8-tetrabenzoate-pyrene and two water molecules as ligands, featuring a large pore size of approximately 31 Å, allowing large phosphonate ester molecules to enter the pores entirely. After dehydration, the exposed Zr serves as highly active Lewis acid sites. Experimental results and theoretical calculations both indicate that the active sites originate from the Lewis acidic Zr4+. Lipase is a type of hydrolase that can catalyze the hydrolysis and esterification of esters. Currently, much research focuses on lipase immobilization technology,75 but the storage stability issues and high cost of natural lipases remain major obstacles to their widespread application. Against this backdrop, developing MOF materials that can mimic lipase activity holds significant research value. Liu et al.76 discovered that UiO-66 and its derivative UiO-66(NH2) exhibit lipase-like activity. UiO-66(NH2) is composed of Zr6 and the ligand NH2-BDC. The exposed Zr clusters activate the carbonyl carbon by attracting electrons from carboxylates and carboxylic acids. Simultaneously, the nucleophilic N atom in the NH2-BDC ligand activates the O atom of alcohol or water molecules, promoting their deprotonation. In the presence of UiO-66(NH2) the ester groups are easily formed. Although the structure of MOFs differs from that of lipases, this synergistic effect is similar to the active sites of natural enzymes. MOF-on-MOF compounds have attracted considerable attention recently due to their rich composition (such as metal centres/organic linkers), structural diversity (such as porosity and surface functionality), synergistic effects and improved sensing performance compared to single units. Xu et al.77 reported a unique core–shell structured MOF-on-MOF composite formed by UiO-66-NH2 and MIL-101(Cr). In addition to the characteristics of the single MOFs, the MOF-on-MOF composites showed significant synergistic effects in the degradation of chemical warfare agent (CWA) simulants. Furthermore, Zr-MOFs can effectively mimic horseradish peroxidase, owing to the similarity between the enzyme cofactors and the structural motifs of Zr-MOFs, such as the defect active sites in Zr6 clusters. However, the activation of these defect active sites, specific active conformations, and reaction mechanisms in these biomimetic reactions remain largely unknown. Xu et al.78 have shown that Zr-based MOFs exhibit ALP-like activity under alkaline environments, and that the activity of these MOFs can be tuned by adjusting the activation pH and reaction pH. This revealed the role of activation procedures, unique active sites and reaction mechanisms in pH-controlled biomimetic aqueous settings. Investigating the activation methods of these basic sequence, the structural information of the active conformation and the transition states in these mimetic reactions could fill a gap in the study of artificial enzymes.
In previous studies, peroxidase mimics constructed from MOF materials exhibited a significant drawback: they tend to deactivate under near-neutral pH conditions. This limitation hinders their widespread application and promotion in the field of biosensing under near-neutral pH conditions. Zheng et al.79 reported that MOF-808 exhibits POD-like activity under near-neutral pH conditions (Fig. 5D). Experiments indicate that this catalytic activity is primarily attributed to its Zr–OH(OH2) groups, which can decompose H2O2 into ˙OH through electron transfer. However, gluconic acid can shield the Zr–OH(OH2) active groups, thereby inhibiting the catalytic activity of MOF-808. Based on this, a glucose sensing colorimetric method was established by combining glucose oxidation with TMB oxidation. Reducing the size of MOFs can significantly enhance their performance and improve sensing sensitivity due to their larger surface area and a higher number of exposed active sites. Wang et al.80 successfully synthesized Zr-MOF nanozymes using polyvinylpyrrolidone (PVP) as a capping agent, enhancing catalytic activity by reducing crystal size to approximately 45 nm. Compared to larger Zr-MOFs, the catalytic activity of the Zr-MOF-PVP nanocomposite was greatly enhanced. Kinetic analysis showed that Zr-MOF-PVP has a much higher affinity for TMB and H2O2 than HRP. Based on this, a simple, rapid, and sensitive colorimetric sensor for H2O2 and phenol was established.
In addition to organophosphorus hydrolases and proteases, carbonic anhydrase (CA) is also a type of hydrolase. Inspired by the composition and structure of carbonic anhydrase II (hCAII), a series of Zn-based MOFs with carbonic anhydrase-like activity83,84 were developed. Wright et al.85 successfully synthesized MFU-4l-(OH) by introducing organic hydroxides into MOFs. This demonstrated the crucial role of Zn–OH groups in mimicking CA activity within MOFs. Compared to three-dimensional MOFs, two-dimensional MOFs possess a larger surface area, thinner thickness, and more accessible active sites. Han et al.86 have produced and characterized Co-ZIF-67, which exhibited superior OXD-like activity due to its two-dimensional sheet-like structure compared to crystalline MOFs (Fig. 5E). This enabled outstanding sensitive colorimetric sensing of glutathione (GSH). Introducing defect structures is also an effective strategy to enhance the catalytic efficiency of MOFs. This can typically be achieved by using structural modifiers, inducing linker deficiency or breakage, and employing solvent competition, leading to unsaturated ligand coordination, promoting the evolution of material morphology, and increasing porosity. Such carefully designed defect structures in MOFs can enhance the contact between active sites and substrates, significantly improving sensor performance. Ren et al.87 successfully introduced controllable defects in ZIFs by incorporating cysteine (Cys) into the mixture of Co2+ and 2-methylimidazole. The study showed that the increased degree of defects significantly enhanced the material's mimic oxidase and mimic laccase activities (Fig. 5F), enabling effective electrochemical detection of uric acid (UA).
Since Alivisatos et al.88 first synthesized enzyme-like Mn3O4via thermal decomposition in 1990, Mn-MOFs have attracted increasing attention. Miao et al.89 studied and prepared Mn-containing mesoporous MOFs (Mn-TMA-MOF, TMA = 1,3,5-benzenetricarboxylate) with OXD-like activity. Mn-TMA-MOF exhibits excellent OXD-like activity due to its structural properties similar to those of natural Mn oxidase. Based on the high stability of Mn-TMA-MOF, a colorimetric sensor for the determination of the total anti-oxidant capacity of fruits has been developed. In recent years, psychrophilic enzymes have garnered increasing attention for their excellent catalytic activity at low temperatures or below freezing points. Chen et al.90 demonstrated the design of manganese-based MOFs (nMnBTC, BTC = 1,3,5-benzenetricarboxylate) as a psychrophilic enzyme mimic, showing nearly no loss of activity in the temperature range of 0 to 45 °C, thus paving the way for sensor applications in low-temperature fields.
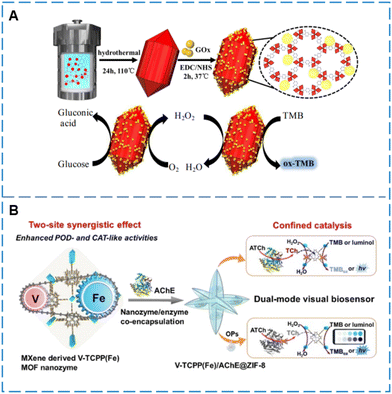
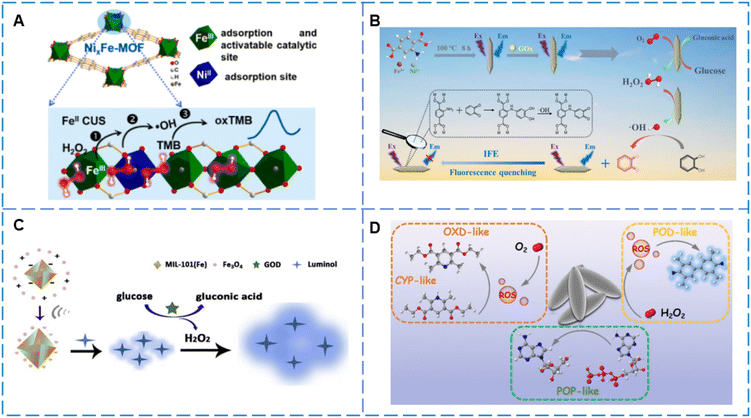
Compared to single-metal MOFs, bimetallic MOFs exhibit potential differences between the two metals, and this heterogeneity is key to the electron transfer process. This not only promotes electron migration efficiency but also provides enhanced driving force for numerous catalytic reactions, thereby significantly improving the overall catalytic performance97,98 and increasing sensing efficiency. Dang99 used a simple one-step hydrothermal method to synthesize hybrid anchored carbon nanotubes (Au/MOFs (Fe, Mn)/CNTs) composed of metal–organic frameworks (Fe, Mn) and Au nanoparticles. Au/MOFs (Fe, Mn)/CNTs exhibited highly enhanced POD-like activity. This is attributed to the increase in active sites, increased partial charge density and electron transfer between the Fermi levels of both MOFs, Au and CNTs, and the synergistic effect of Fe and Mn. Using glucose oxidase (GOx), a glucose detection system cascaded with the nanocomposite was developed. Additionally, embedding bimetallic centers within MOF frameworks possessing morphological advantages can significantly enhance the mimic enzyme activity, thereby improving sensor performance. Li et al.100 constructed bimetallic active centers using hollow MOFs with larger specific surface area, shorter interface transport distances, and more exposed active sites, effectively achieving the sensing of acetylcholinesterase (AChE).
On the other hand, constructing bimetallic active centers can enhanced the density of active catalytic sites, thereby significantly enhancing the catalytic efficiency of the material.101,102 Kulandaivel et al.103 reported a bimetallic nanozyme MOF-919 (Fe–Cu) with both OXD-like and POD-like activities (Fig. 5G(a)). In this system, OPD used as a substrate for chromogenic (Fig. 5G(b)), and its oxidation process can be divided into two stages: first, Cu-SBUs exhibit OXD-like activity; subsequently, Fe-SBUs demonstrate POD-like activity, thereby enhancing substrate oxidation. Based on this, a colorimetric sensor for adrenaline was developed.
Unlike natural enzymes, most non-protein artificial enzymes lack recognition sites for specific substrates. This limitation prevents them from performing specific catalysis. The limitation of the selectivity of MOFs nanozymes has been hindering their further application in the field of sensing. To overcome this problem, the researchers introduced external molecular recognition units (molecular imprinting technology), an innovative strategy that can effectively enhance the recognition of analytes by MOFs nanozymes, thus advancing the development of MOFs-based sensor technology. Zhang et al.104 prepared a molecularly imprinted polymer nanozyme, MIL-101(Co,Fe)@MIP, with bimetallic active centers and POD-like activity. Compared to single-metal nanozymes, the bimetallic structure significantly improves its POD-like activity by adjusting electronic and surface properties (Fig. 5H). By allowing vanillin to enter specific imprinted cavities and block molecular channels on the surface of MIL-101(Co,Fe)@MIP, proportionate fluorescence and colorimetric dual-mode detection of vanillin in aqueous solution was achieved. In addition, intrinsic engineering to modulate the active site of nanozymes through coordination environments and doping has been successful.92,94–96 However, these approaches are either hampered by limited types of artificial recognition units or can only be engineered for specific types of catalysts. Therefore, the development of non-protein artificial enzymes with high specificity remains a challenge.
Polymetallic MOF nanozymes have emerged as a rapidly expanding branch within the nanozyme field, offering tremendous potential for sensing applications due to their high catalytic activity, tunable electronic and structural properties, and vast compositional versatility. Compared to mono- and bimetallic MOF nanozymes, polymetallic variants significantly increase the number of exposed active sites by incorporating additional metals into secondary building units (SBUs).105 This design leverages the synergistic effects of diverse metal elements, enhancing catalytic performance and accelerating charge transfer between metals.106 Moreover, the introduction of hydrolysis-resistant metals into the framework improves the stability of pristine MOFs. The inherent robustness of polymetallic systems not only provides excellent durability but also significantly enhances catalytic efficiency, making them highly effective for sensor applications.107 A notable example is the pioneering colorimetric sensing platform developed by Hou et al.108 based on a trimetallic MOF nanozyme (ZnCo-ZIFs@MIL-101(Fe)). This system capitalizes on the electronic hybridization and synergistic interactions among Fe3+, Co2+, and Zn2+ metal centers, enabling exceptional peroxidase-like catalytic activity. The platform offers ultra-sensitive, cost-effective, portable, and rapid on-site detection of glyphosate. Despite the substantial progress achieved with polymetallic MOF nanozymes, several challenges persist. Conventional preparation methods limit scalability and high-throughput production, posing significant barriers to their broader application. Optimizing the activity and selectivity of polymetallic MOF nanozymes remains critical. Furthermore, many aspects of their structural arrangements, functions, and mechanisms of action are not fully understood, highlighting the urgent need for further research to unlock their full potential.
2.2 Ligands as active sites
The organic ligands in MOFs can also mimic the active centers of natural enzymes, endowing them with enzyme-like activity. These organic ligands act as electron transfer mediators, capable of receiving and transmitting substrate electrons, allowing MOF sensors to quickly respond to substrate changes, thereby improving detection speed and accuracy. Porphyrin-based ligands are particularly outstanding in this regard.109,110 Additionally, various active functional groups on organic ligands, such as amino, amide, pyridyl, and bipyridy groups, can serve as catalytic active sites. By rationally designing and selecting organic ligands, MOF sensors can exhibit excellent performance in the detection of various analyte molecules.As a highly stable ligand, porphyrin can form complexes with metal ions, some of which exhibit activities similar to those of natural peroxidases.111–116 Additionally, the C–O–M (M represents metal) bonds formed between porphyrin and metal nodes act as channels for electron transfer, significantly facilitating the charge transfer process and further enhancing the enzyme-like activities in these systems. In 2012, Feng et al.117 adopted Fe-TCPP (TCPP = (4-carboxyphenyl) porphyrin) as a heme-like ligand and utilized highly stable Zr6 clusters as nodes. They successfully assembled PCN-222(Fe) with POD-like activity. PCN-222(Fe) can catalyze the oxidation of various substrates, exhibiting superior substrate binding affinity and catalytic activity, and outperforms hemin in aqueous media. To determine whether the catalytic activity of these MOFs originates from the metal nodes or their heme-like metalloporphyrin ligands, Cheng et al.114 has prepared seven types of 2D MOF nanosheets (Fig. 6A), and demonstrated that the Fe-TCPP ligand is critical in conferring POD-like activity to the MOF nanozymes, while the metal nodes are primarily responsible for structural construction. Not only can iron porphyrin be used as a ligand for MOF nanozymes, but its derivative hemin can also serve the same function. Liu et al.118 were the first to use hemin as a ligand and copper as the metal ion to construct Cu-hemin MOFs with POD-like activity (Fig. 6B(a)). The porous structure prevents hemin from self-degradation and aggregation, thereby enhancing its activity (Fig. 6B(b)). With the assistance of glucose oxidase, Cu-hemin MOFs were used as catalysts to develop a highly sensitive method for glucose detection, expanding the functionality of MOFs in biosensor applications.
 | ||
| Fig. 6 (A) Scheme showing the surfactant-assisted bottom-up synthesis of 2D MOF nanosheets. (Reproduced from ref. 114.) (B) (a) Synthetic route for Cu-hemin MOFs and detection mechanism for H2O2 and glucose sensing. (b) UV–Vis absorption spectra of TMB in the presence of Cu-hemin MOFs and H2O2 with different concentrations (reproduced from ref. 118). (C) (a) Illustrates the proposed mechanism of the H2O2-TMB reaction catalyzed by the Zr-MOFs peroxidase mimic. (b) Compares UV-vis profiles of various systems (reproduced from ref. 121). | ||
In recent years, a large number of organic compounds have been studied as enzyme mimetics, among which those with abundant orbital holes and numerous free π-electrons are particularly notable, such as bipyridine groups. In such structures, electrons from donors can be easily captured. Subsequently, these electrons are transferred to other acceptors, showcasing its central role in electron transfer.119,120 Wang et al.121 reported a Zr-MOFs with POD-like activity, in which 2,2′-bipyridine-4,4′-dicarboxylic acid (BPDCA) can catalyze the oxidation of TMB in the presence of H2O2 (Fig. 6C(a)). This indicates that the POD-like activity mainly originates from the BPDCA ligand, rather than the Zr nodes (Fig. 6C(b)). Based on this, a colorimetric sensor for quantitative detection of phosphorylated proteins was developed.
2.3 Post-synthetic modifications to increase the active site
MOFs can achieve functional modifications through their metal nodes/clusters and organic ligands, as well as by incorporating guest molecules into the pore space. This is usually accomplished through direct modification or post-synthetic modification. However, introducing functional groups directly during MOF synthesis is challenging. Additionally, directly introducing functional groups onto organic ligands often leads to solubility issues or coordination with metal ions, thereby hindering the formation of MOFs. Post-synthetic modification methods can introduce various functional groups without destroying the MOFs framework, thereby enhancing the sensitivity and selectivity of the sensors.2.3.1.1 Metal nodes modification to increase active sites. The CUSs of MOFs can be modified with active species through chemical bonding. Precise modification of the metal nodes can effectively regulate the electronic structure and surface properties123 of MOF nanozymes, significantly enhancing the catalytic efficiency and selectivity of the sensors. It also allows fine-tuning of the pore size,124 surface charge, and hydrophilicity/hydrophobicity of MOFs, thereby optimizing the response time and detection accuracy of the sensors. Cui et al.125 obtained PB/MIL-101(Fe) with highly efficient POD-like catalytic activity by coordinating the Fe3+ vacancies on the surface of MIL-101(Fe) with the CN residues on the main chain of Prussian blue (PB). The MIL-101(Fe) component helps expand the pore space for adsorbate storage, while the presence of PB increases electron transfer between the catalyst and the substrate. A sensitive colorimetric biosensor platform was established on this basis. Valekar et al.122 prepared MIL-100(Fe) functionalised with aliphatic diamines by grafting aliphatic diamines onto the coordinatively unsaturated metal sites of MIL-100(Fe). Due to the synergistic effects of enhanced negative potential from grafted diamines and precise molecular size control, TMBDA-MIL-100(Fe) exhibits excellent POD-like activity, enabling fluorescent sensing of choline and acetylcholine. Furthermore, MOF nanozymes tend to aggregate/precipitate in aqueous solution, with weak colloidal stability, which affects their application in aqueous media. Mao et al.126 covalently connected MIL-53(Fe) with the G4-forming sequence (F3TC), where F3TC itself was covalently connected to its cofactor hemin. The synergistic effect of the active sites between MIL-53(Fe) and G4-hemin endowed the MIL-53(Fe)/G4-hemin compound with outstanding POD-like activity and superior robustness (Fig. 7A). Utilizing these properties, an ALP sensor suitable for human plasma samples was designed, expanding its range of applications.
 | ||
| Fig. 7 (A) Effects of different MOFs on catalytic activity with F3TC-hemin. (Reproduced from ref. 126.) (B) Schematic illustration of the UiO-66-Se catalysts obtained by PSM performed on UiO-66-NH2. (Reproduced from ref. 127.) (C) Schematic illustration of protamine and trypsin sensing based on heparin boosted laccase-like activity of Bpy–Cu. (Reproduced from ref. 130.) (D) Schematic diagram showing the tandem catalysis of GOx@HP-PCN-224(Fe). (Reproduced from ref. 138.) (E) (a) Schematic illustration of the AuPt/ZIF-8-rGO catalysts. (b) AuPt/ZIF-8-rGO catalyzing the oxidation of A OPD, B TMB, and C ABTS to produce different colors in the presence of H2O2. (c) The absorption spectra and digital photos of different reaction system: (A) OPD; (B) OPD + H2O2; (C) OPD + H2O2 + GO; (D) OPD + H2O2 + ZIF-8-GO; (E) OPD + H2O2 + AuPt/ZIF-8-rGO. (Reproduced from ref. 143.) | ||
2.3.1.2 Ligand modification to increase active sites. Functional materials (such as organic molecules, metal complexes, and chiral molecules) can also be introduced into the ligands of MOFs through post-synthetic modification. This method helps to optimize the interaction between MOF sensors and analyte molecules, thereby enhancing their recognition and capture abilities. Zhou et al.127 developed MOFs with glutathione POD-like activity by grafting phenyl selenyl bromide (PhSeBr) onto UiO-66-NH2 (Fig. 7B), where PhSe-NH acted as a functional donor and catalytic center. MOFs with high specific surface area and uniform porosity provide more catalytic active centers. The porphyrin ring structure of metalloporphyrins is easily destroyed during oxidation, leading to rapid loss of activity, thus limiting their practical application. To address this issue, Liu et al.128 synthesized Fe-MOFs. The covalent bonds between the catalytic sites in the framework confer high stability and repeatability to FePPOPs–SO3H, improving the stability of the sensor and enabling colorimetric detection of H2O2 and glucose. Metal ions can also be introduced into MOFs via post-synthetic modification.109 Chen et al.129 synthesized UiO-type metal–organic framework nanoparticles (NMOFs) and obtained Cu2 ± NMOFs by post-synthetically modifying the bipyridine ligands with Cu2+, exhibiting excellent peroxidase-like activity with Cu2+/bipyridine ligands as the active centers. Using Cu2 ± NMOFs to catalyze chemiluminescence in the presence of luminol/H2O2, a chemiluminescent sensor for glucose was developed.
2.3.1.3 MOFs unit modification increase active sites. By utilizing non-covalent interactions, such as electrostatic forces and π–π stacking, active molecules can be stably anchored onto MOFs units. This method can significantly enhance the application performance of MOFs in analytical sensing. Li et al.130 discovered that the laccase mimic Bpy–Cu can catalyze the oxidation of 2,4-dichlorophenol and react with 4-aminoantipyrine (4-AP) to form a red product (Fig. 7C). Through electrostatic interaction, Bpy–Cu binds with negatively charged heparin to form a Bpy–Cu/Hep complex, enhancing the adsorption of positively charged 4-AP and improving catalytic efficiency. On this basis, a new sensing strategy for protamine and trypsin was proposed. Other charged substances can also be used to modify the surface of MOFs. Dong et al.131 synthesized glycine-MIL-53(Fe) by simply mixing water-dispersed MIL-53(Fe) with glycine through electrostatic interaction. Due to the increased affinity between TMB and glycine-MIL-53(Fe), its catalytic activity and stability are significantly higher than that of MIL-53(Fe). A method for the detection of H2O2 and glucose has been developed using these characteristics. Electrostatic interaction is a primary method for modifying MOFs units. Additionally, π–π interaction can be used for functional modification of MOFs units. Shu et al.132 immobilized Hemin on 2D Ni-MOF nanosheets through π–π interaction. Electrocatalytic reduction of H2O2 demonstrated that the compound material has strong catalytic activity. The enhanced catalysis is due to the immobilisation process, which prevents the self-aggregation of Hemin. Based on this, a high-performance electrochemical H2O2 biosensor was developed.
2.3.2.1 Enzyme@MOF nanozymes. Although natural enzymes exhibit high selectivity and catalytic efficiency in specific reactions, their environmental stability and low reusability limit their widespread application. By immobilizing natural enzymes onto inorganic carriers, these protein molecules can be effectively protected, preventing environmental factors from damaging enzyme activity. This not only enhances the catalytic activity of the enzymes but also significantly improves the stability of sensors in complex environments. Additionally, the reusability and recyclability of immobilized enzyme sensors are significantly improved, thereby reducing the cost of sensor usage and minimizing environmental impact.
Cascade reactions involve a series of continuous signals triggered by enzymatic reactions and their amplification effects. By combining MOFs with natural enzymes, such enzyme cascade reaction systems can be meticulously designed. This approach not only improves the efficiency and selectivity of the reactions but also provides a novel and efficient platform for enzyme-based complex reaction pathways.133–136 In Zhong's research,137 a multi-enzyme system was constructed by combining MOF-545(Fe), which possesses POD-like activity, with biological enzymes. Due to the large pore volume of MOF-545(Fe), GOx can easily enter its channels through simple direct mixing. MOF-545(Fe) not only serves as a carrier for immobilizing enzymes but also has catalytic activity for cascade reactions in conjunction with natural enzymes. On this basis, a colorimetric biosensor for the rapid sensing of glucose was realized. However, such a simple and direct post-synthesis encapsulation method is limited by the porosity of the MOFs, and only MOFs with larger pore diameters, such as ZIF-8 and MIL-100(Fe), can be used. For MOFs with smaller pore diameters, auxiliary measures can be taken during synthesis to control porosity, such as adding larger macromolecules to enlarge the pores. Liu et al.138 prepared biomimetic MOFs HP-PCN-224(Fe) with a hierarchical porous structure by adding dodecanoic acid (DA) during the synthesis of PCN-224(Fe). Subsequently, DA was removed through hydrochloric acid treatment, creating larger mesoporous spaces to effectively encapsulate GOx (Fig. 7D). HP-PCN-224(Fe) not only serves as an immobilization matrix for enzymes but also acts as an effective enzyme mimic, synergistically catalyzing cascade reactions with the immobilized natural enzyme, effectively achieving colorimetric detection of UA. However, due to relatively larger pores, hierarchical porous MOFs are prone to leakage. Zhao et al.139 modified the synthesized HP-MIL-88B with boric acid and then encapsulated glucose oxidase in the pores of the MOFs, forming specific interactions between the enzyme and the carrier. This approach prevents enzyme leakage, achieves high encapsulation efficiency, and improves the stability of the sensor. Unlike the synthesis of HP-MOFs to enlarge pore sizes, de novo encapsulation methods such as co-precipitation can encapsulate natural enzymes of any shape and size. Wang et al.140 used the co-precipitation technique to co-immobilize NiPd hollow nanoparticles and GOx in ZIF-8, resulting in the formation of GOx@ZIF-8(NiPd) nanoflowers that exhibit both POD-like activity of NiPd and enzymatic activity of GOx. The GOx@ZIF-8(NiPd) modified electrode retains the bioactivity of GOx and exhibits high electrocatalytic activity for the oxygen reduction reaction, making it also applicable for the electrochemical sensing of glucose.
2.3.2.2 Inorganic nanozymes@MOF nanozymes. Inorganic nanozymes have been demonstrated to mimic the catalytic activity of natural enzymes while avoiding the stability issues associated with biological enzymes. However, standalone inorganic nanozymes often face problems such as aggregation and easy deactivation, limiting their applicability. The crystalline porous structure of MOFs provides excellent conditions for stabilizing inorganic nanozymes, preventing aggregation and ensuring high activity and stability during sensing processes.141 Moreover, the controlled integration of inorganic nanozymes within MOFs can endow the inorganic nanozymes@MOFs with synergistic catalysis effects, thereby accelerating detection reaction rates and improving detection accuracy.
In recent years, noble metal nanoparticles based on MOF nanozymes as carrier materials have garnered widespread attention. Common noble metal nanoparticles include PtNPs, AuNPs, AgNPs, etc.142 Zhang et al.143 reported AuPt/ZIF-8-rGO, which exhibited enhanced POD-like activity due to the excellent dispersion of AuPtNPs (Fig. 7E(a)), the bimetallic synergistic effect of Au and Pt, and the strong metal–support interactions with ZIF-8-rGO (Fig. 7E(b)). AuPt/ZIF-8-rGO showed superior performance in the actual sensing of H2O2 (Fig. 7E(c)). A novel carbon nanomaterial, carbon dots can also be embedded into the pores of MOF structures, exposing and providing more diverse active sites, accelerating electron transfer and ion diffusion. Wang et al.144 designed and synthesized CDs@ZIF-8 nanocomposites. Their synergistic action endowed the materials with excellent POD-like activity, which can be used for the colorimetric detection of H2O2 and GSH, expanding the application of MOFs in bioanalysis.
2.4 Active sites of MOFs-derived nanozyme
MOFs and their composites face significant limitations in practical applications due to issues such as easy decomposition, structural collapse, or limited solubility. To address these shortcomings, MOFs can be used as precursors or sacrificial templates through a one-step direct carbonization/oxidation or an indirect post-treatment process to synthesize various MOF derivatives, such as single-atom nanomaterials, metal/carbon nanomaterials, and metal oxide/carbon materials. These derivatives retain the mesoporous properties of MOFs, and their mesoporous and multi-channel structures ensure sufficient contact between the substrate and the material surface, thereby enhancing the sensitivity and detection efficiency of sensors. Furthermore, the improved stability and activity of these derivative materials ensure the reliability and longevity of sensors in complex environments.2.4.1.1 Fe single-atom nanozymes. In natural cytochrome P450 peroxidase, the Fe(III) metal ion is located at the centre of the porphyrin nucleus, coordinating with the four nitrogen atoms in the porphyrin ring and another nitrogen atom from a cysteine group. MOFs serve as excellent precursors for constructing such heterogeneous atomic-doped carbon materials, particularly for single-atom nanomaterials with the common M–N–C structure. The M–N–C structure has a partially unsaturated coordination environment and a unique electronic structure compared to metal nanoparticles, making it an ideal catalyst for various reactions. Based on these characteristics, an increasing number of studies on Fe-based single-atom nanozymes have been reported.145–147 Niu et al.148 developed a single-atom nanozyme (SAN) composed of Fe–N–C, exhibiting POD-like activity (Fig. 8A). The SAN is made up of atomically dispersed Fe–Nx groups supported on a porous carbon that is derived from MOFs. Because of the 100% atom dispersion of the active sites and the large surface area of the porous scaffold, the Fe–N–C SAN exhibits excellent catalytic activity, effectively enabling biosensing of butyrylcholinesterase (BChE). Meanwhile, Zhu et al.149 designed and synthesized Fe–N5 decorated graphene nanosheets (Fe–N5/GN SAC) with single-atom Fe sites, which efficiently decompose H2O2 to generate hydroxyl radicals, showcasing superior POD-like activity. Single-atom nanozymes with dual or multiple enzyme activities are particularly noteworthy because they mimic the functions of multiple enzymes within a single nanoscale entity, allowing for the processing of various substrates or the promotion of different types of chemical reactions under the same catalytic conditions. Lu et al.146 prepared Fe-TPP⊂rho-ZIF by a mechanical synthesis method and pyrolyzed it to obtain the carbon-supported atomically dispersed Fe–N4 (Fe–N/C SACs) composite material. This material exhibits multiple enzyme-mimicking activities, including GPx-like, CAT-like, POD-like, and OXD-like activities. As a result of its activity, Fe–N/C SAC has been successfully used for the scavenging of intracellular ROS.
 | ||
| Fig. 8 (A) Illustrates the preparation process of the Fe–N–C SAN. (Reproduced from ref. 148.) (B) Schematic illustration of the synthesis of Cu–N–C SAzymes. (Reproduced from ref. 150.) (C) Illustration of CuNPs@C catalyzed oxidation of TMB in the presence of H2O2 and the inhibition effect of AA, as demonstrated by the color change. (Reproduced from ref. 158.) (D) Illustration of detecting DA by Co3O4 HNCs with the colorimetric methods. (Reproduced from ref. 163.) | ||
2.4.1.2 Cu single-atom nanozymes. Despite the considerable advancement in the nanozyme research field brought about by the introduction of single-atom nanozymes (SAzymes), new challenges have also emerged. Currently, most studies focus on iron-based SAzymes. However, in certain application scenarios, the multi-enzyme activity of highly active iron-based SAzymes may pose limitations. For example, in the field of sensing, although nanozymes with POD-like activity are widely used, their OXD-like activity may lead to undesirably high background signals. Therefore, exploring and developing SAzymes based on other metals has become an effective strategy. Wu et al.150 used a salt-template strategy to prepare single-atom POD-like nanozymes (Cu–N–C) with high Cu sites concentration. Thanks to the dense active Cu atoms, Cu–N–C SAzymes exhibited excellent POD-like activity, with CuN4 acting as the active center (Fig. 8B). On this basis, a three-enzyme cascade reaction system was constructed for the colorimetric detection of acetylcholine and organophosphorus pesticides. Meanwhile, Li et al.151 developed a Cu2O–CDs–Cu tri-component oxidase catalyst, which showed photocatalytic OXD-like activity and high reaction rates when catalyzing the oxidation of OPD.
2.4.1.3 Other metal-based single-atom nanozymes. Mn single-atom nanozymes possess unique advantages such as high catalytic activity, high selectivity, strong stability, low cost, and environmental friendliness, making them outstanding in biosensing and analysis. They not only significantly improve detection sensitivity and accuracy but also maintain high catalytic performance under harsh conditions, making sensors more reliable and efficient in low-concentration analytes detection and complex environmental applications. However, exploration of single-atom nanozymes with manganese as the active metal center is relatively limited, with current research mainly focusing on coordination structures formed by Mn and N.152–154 Doping with other types of heteroatoms also affects the catalytic activity of manganese-based single-atom nanozymes. Feng et al.155 synthesized Mn-centered single-atom nanozymes by doping N, P, and S atoms, significantly enhancing their POD-like activity. This enhancement is attributed to the synergistic effect of N, P, and S promoting their catalytic activity.
Natural halogen peroxidases (HPOs) can efficiently catalyze the reaction between two-electron halides and H2O2, producing hypohalous acids with antibacterial and antiviral activity. However, natural enzymes face issues such as high extraction costs, insufficient biological stability, and low reaction efficiency in saline environments, which limit their practical applications. Therefore, developing stable and economical enzyme-like systems has become an urgent need. Wang et al.156 reported HPO-like nanozymes (Mo SA–N/C) with Mo single-atom active sites, which can inhibit biofouling in the actual marine environment. Catalase can efficiently catalyze the decomposition of H2O2, converting it into water and oxygen. This process is crucial in reducing oxidative stress and protecting cells from H2O2-induced damage in biological systems. Chen et al.157 successfully synthesized single-atom Co nanozymes (Co-N3PS SAzyme) with a CoN3PS active configuration using an Ordered Structure Directing Coordination (OSDC) strategy, demonstrating exceptional CAT-like activity. This approach lays the groundwork for replacing conventional enzymes with single atomic nanomaterials with well-defined atomic structures and electronic coordination environments.
Tan et al.158 used the [Cu3(BTC)2] precursor to prepare Cu NPs@C nanocomposites via a one-step pyrolysis method. Thanks to the spatial confinement of the MOF precursors, Cu NPs are highly dispersed within the carbon matrix, exhibiting excellent POD-like activity (Fig. 8C). Due to the absence of stabilizers/coupling agents on the Cu NP surfaces, Cu NPs@C shows higher catalytic activity towards H2O2 than HRP. This has been the basis for the development of a colorimetric AA detection method. Compared to single metals, bimetallic systems often provide superior properties, including enhanced stability and improved catalytic activity. Wu et al.159 obtained FeCo bimetallic-doped carbon composites, FeCo@C, by carbonizing FeCo-ZIF, which possess excellent OXD-like and POD-like mimic activities. The unique dual-enzyme catalytic properties of FeCo@C can be easily tuned and used in a dual-functional colorimetric platform for the visual detection of hydroquinone (HQ) and H2O2 based on their respective OXD-like and POD-like activities.
3. Sensing mechanism based on MOF nanozymes
By studying the intrinsic relationship between the activity of nanozymes and their components, MOF-based nanozymes can be constructed and applied in various fields. Additionally, by constructing multi-enzyme systems and employing strategies to enhance activity, MOF-based nanozymes have the potential to replace natural enzymes in many areas. According to recent literature, their applications are mainly classified into three areas: analytical sensing, environmental treatment, and disease therapy. Among these, MOF-based nanozymes have been widely used in the field of analytical sensing, as summarized in Table 1. Generally, the sensing mechanisms based on MOF-based nanozymes can be categorized into the following five types: (1) the analyte acts directly as the substrate for the MOF-based nanozyme catalytic reaction: MOF-based nanozymes are used directly to detect enzyme substrates (e.g., phenols, H2O2) and related substrate molecules (e.g., glucose); (2) the analyte affects the active sites of MOF-based nanozymes: the activity of MOF-based nanozymes can be easily influenced by external molecules (e.g., inorganic ions and DNA), enabling the detection of these substances by adjusting the nanozyme activity; (3) dual active site cascade catalysis of MOF-based nanozymes: two different catalytic active sites are integrated within a single nanostructure, achieving efficient analyte molecules recognition through dual active site cascade catalysis; (4) the analyte affects the catalytic reaction of MOF-based nanozymes: substances like biothiols, ascorbic acid (AA), and dopamine (DA) significantly impact the nanozyme catalytic system, allowing these substances to be detected by MOF-based nanozymes; (5) signal tagging based on MOF-based nanozymes: as potential replacements for natural enzymes, MOF nanozymes can also be used for signal amplification in immunoassays and aptamer sensors.| Sensing mechanism | MOFs | Source of activity | Active site | Sensing methods | Analyte | Linear range | LOD | Ref. |
|---|---|---|---|---|---|---|---|---|
| Inhibition of catalytic reactions | MIL-53(Fe) | Fe nodes | Metal nodes | Colorimetric | AA | 28.6–190.5 μM | 15 μM | 26 |
| Dual active site cascade catalysis | Fe-MIL-88NH2 | Fe nodes | Metal nodes | Colorimetric | Glucose | 2.0 × 10−6–3 × 10−4 M | 4.8 × 10−7 M | 21 |
| Dual active site cascade catalysis | Cu-MOF | Cu nodes | Metal nodes | Chemiluminescence | Glucose | 6.0 × 10−7–2.0 × 10−5 M | 8.7 μM | 47 |
| Dual active site cascade catalysis | Cu FMA | Cu nodes | Metal nodes | Colorimetric | Glucose | 2.7–54.6 μM | 1.1 μM | 54 |
| Directly as a substrate for catalytic reactions | Epinephrine | 0.01–0.8 mM | 2.28 × 10−7 M | |||||
| Directly as a substrate for catalytic reactions | MOF-919(Fe–Cu) | Fe/Cu nodes | Metal nodes | Colorimetric | Epinephrine | 1–100 μM | 0.298 μM | 103 |
| Directly as a substrate for catalytic reactions | MIL-88 | Fe nodes | Metal nodes | Colorimetric | H2O2 | 2.0 × 10−6–2.03 × 10−5 mol L−1 | 5.62 × 10−7 mol L−1 | 22 |
| Directly as a substrate for catalytic reactions | MIL-68 | Fe nodes | Metal nodes | Colorimetric | H2O2 | 3.0–40 μM | 0.256 μM | 24 |
| Directly as a substrate for catalytic reactions | QHKUST-1 | Cu nodes | Metal nodes | Electrochemical | H2O2 | 1 μM–11 mM | 0.3 mM | 35 |
| Directly as a substrate for catalytic reactions | MOF-818 | Cu nodes | Metal nodes | Colorimetric | H2O2 | 0.0133–10 mM | 15.37 μM | 46 |
| Electrochemical | 10–166 mM | 53.05 μM | ||||||
| Directly as a substrate for catalytic reactions | Ce-MOF | Ce nodes | Metal nodes | Fluorescence | H2O2 | 200–1500 μM | 10 μM | 58 |
| Directly as a substrate for catalytic reactions | Zr-MOF-PVP | Zr nodes | Metal nodes | Colorimetric | H2O2 | 10–800 μM | 2.76 μM | 80 |
| Directly as a substrate for catalytic reactions | Co/Mn-MOF | Co/Mn nodes | Metal nodes | Colorimetric | H2O2 | 1–100 μM | 0.85 μM | 93 |
| Inhibition of catalytic reactions | Ce–BPyDC | Ce nodes | Metal nodes | Colorimetric | AA | 1–20 μM | 0.28 μM | 66 |
| Dual active site cascade catalysis | TMBDA-MIL-101(Fe) | Fe nodes | Metal nodes | Fluorescence | ACh | 0.01–100 μM | 8.9 nM | 122 |
| Directly as a substrate for catalytic reactions | Ce-HMMOF | Ce nodes | Metal nodes | Colorimetric | ATP | 0.5–100 mM | 0.28 mM | 60 |
| Inhibition of catalytic reactions | D-ZIF-67 | Co nodes | Metal nodes | Colorimetric | GSH | 0.5–10 mM | 229.2 nM | 61 |
| Influence active site | Ce-MOF | Ce nodes | Metal nodes | Colorimetric | Cys | 165–335 μM | 0.15 μM | 56 |
| Dual active site cascade catalysis | Cu-hemin MOFs | Hemin | Ligand | Colorimetric | H2O2 | 1.0 IM–1.0 mM | 0.42 IM | 118 |
| Directly as a substrate for catalytic reactions | FePCN | TCPP(Fe) | Ligand | Colorimetric | H2O2 | 5–200 μM | 0.48 μM | 113 |
| Inhibition of catalytic reactions | PSMOF | Zn nodes/Ru(bpy)32+ | Ligand | Colorimetric | GSH | 0–20 μM | 0.68 μM | 193 |
| Influence active site | Zr-based MOF | BPDCA | Ligand | Colorimetric | Protein | 0.17–5.0 μg mL−1 | 0.16 μg mL−1 | 121 |
| Directly as a substrate for catalytic reactions | PB/MIL-101(Fe) | Fe nodes/PB | Functional modifications | Colorimetric | H2O2 | 2.40–100 mM | 0.15 mM | 125 |
| Directly as a substrate for catalytic reactions | FePPOPs–SO3H | Porphyrin/SO3H | Functional modifications | Colorimetric | H2O2 | 50–1800 μM | 26.70 μM | 128 |
| Directly as a substrate for catalytic reactions | AuPt/ZIF-8-rGO | AuPt NPs | Functional modifications | Electrochemical | H2O2 | 100 nM–18 mM | 19 nM | 143 |
| Influence active site | Bpy-Cu | Cu nodes/Hep | Functional modifications | Colorimetric | Trypsin | 0.01–10 μg mL−1 | 3.3 ng mL−1 | 130 |
| Dual active site cascade catalysis | MIL-53(Fe)/G4-hemin | Fe nodes/G4-hemin | Functional modifications | Colorimetric | ALP | 0–70 U L−1 | 0.02 U L−1 | 126 |
| Inhibition of catalytic reactions | CDs@ZIF-8 | CDs | Functional modifications | Colorimetric | GSH | 0–100 μM | 1.04 μM | 144 |
| Dual active site cascade catalysis | GOx@HP-MIL-88B-BA | Fe nodes/GOx | Functional modifications | Colorimetric | Glucose | 2–100 μM | 0.98 μM | 139 |
| Dual active site cascade catalysis | CoxOyHz@ZIF-67 | Co nodes/CoxOyHz | Functional modifications | Photoelectrochemistry | Glucose | 0.1 μM–1 mM | 0.03 μM | 185 |
| Dual active site cascade catalysis | GOx@ZIF-8(NiPd) | NPs/GOx | Functional modifications | Colorimetric | Glucose | 0.01–0.3 mM | 9.2 μM | 140 |
| Electrochemical | 0.1–1.7 mM | |||||||
| Dual active site cascade catalysis | Uricase@HP-PCN-224(Fe) | Fe nodes/uricase | Functional modifications | Colorimetric | UA | 5–100 μM | 1.8 μM | 134 |
| Signal label | 2D MOF | Au NPs/Ab2 | Functional modifications | Electrochemical | S. aureus | 10–7.5 × 107 CFU mL−1 | 6 CFU mL−1 | 200 |
| Inhibition of catalytic reactions | Cu NPs@C | Cu NPs | Derivatization of MOFs | Colorimetric | AA | 10 μM–1 mM | 1.41 μM | 158 |
| Inhibition of catalytic reactions | Fe–N–C SAN | Fe–Nx | Derivatization of MOFs | Colorimetric | BChE | 0.1–10 U L−1 | 0.054 U L−1 | 148 |
| Inhibition of catalytic reactions | Co3O4 HNCs | Co3O4 NPs | Derivatization of MOFs | Colorimetric | DA | 0.02–3.5 μM | 0.015 μM | 163 |
3.1 Directly as substrate for catalytic reactions
When the analyte substance directly acts as a substrate, it can undergo a specific catalytic reaction with MOF-based nanozymes. During this process, the changes resulting from the catalytic reaction are converted into detectable analytical signals, primarily due to the active sites in the MOF-based nanozymes. For example, in the detection of H2O2, it can act as a substrate for MOF-based nanozymes, producing characteristic signal-generating products such as O2 or compounds with color changes under catalysis. The detection system quantifies the concentration of H2O2 by monitoring the amount of these products or the intensity of the signal generated.H2O2 is one of the most important reactive oxygen species in the human body, associated with diseases such as asthma, inflammatory arthritis, atherosclerosis, and neurodegenerative conditions. Therefore, developing rapid and sensitive methods for detecting H2O2 is crucial. Currently, colorimetric methods79,80,167–169 are widely used for H2O2 detection, with the advantage of detecting target analytes based on color changes, along with the benefits of visual inspection and easy readability. Cheng et al.98 successfully prepared NixFe-MOF, which exhibits POD-like activity. Its NiII nodes are used for substrate adsorption, while the FeII/III nodes enable reversible catalysis (Fig. 9A). A colorimetric sensor for H2O2 was developed on this basis, with a detection range of 1–80 μM and a limit of detection (LOD) as low as 0.59 mM. Compared to colorimetric methods, fluorescence detection offers higher sensitivity and specificity. Furthermore, Mu et al.170 suggested a fluorescence detection technique for H2O2 based on an aminated Fe–Ni bimetallic MOFs nanozyme (Fe3Ni-MOF–NH2) with POD-like activity (Fig. 9B). This method utilizes metal active sites to catalyze H2O2 into ˙OH, which subsequently converts catechol to o-quinone. The o-quinone continues to react with the amino groups in the MOFs, causing fluorescence quenching and achieving a LOD of 5 nM. Additionally, chemiluminescence (CL) systems, which convert chemical energy into light signals, have been widely used in H2O2 detection due to their low detection limits, broad calibration range, lack of need for an excitation light source, simple instrumentation, and ease of operation. Tang et al.171 developed an ultrasensitive H2O2 chemiluminescence analytical technique based on Fe3O4/MIL-101(Fe). Upon addition of H2O2, Fenton or Fenton-like reactions are triggered, producing a large amount of ROS radicals in the Fe3O4/MIL-101(Fe)-luminol system, significantly enhancing the CL signal (Fig. 9C). This technique achieved H2O2 detection in the range of 5–100 nM with a detection limit of 3.7 nM.
 | ||
| Fig. 9 (A) Proposed mechanism of catalytic sites and adsorption sites activated by bimetallic NixFe-MOF nanozymes. (Reproduced from ref. 98.) (B) Schematic diagram of the synthesis and fluorescence detection strategy of Fe3Ni-MOF–NH2/GOx. (Reproduced from ref. 170.) (C) Schematic illustration of the preparation process for the Fe3O4/MIL-101(Fe) and CL method for H2O2 and glucose detection. (Reproduced from ref. 171.) (D) Multienzyme-like activities of FePCN. (Reproduced from ref. 113.) | ||
Ma et al.172 constructed an electrochemical H2O2 sensor based on Cu-TCPP/g-quadruplex hemoglobin nanocomposites by assembling a Cu-TCPP MOFs nanosheet with a g-quadruplex DNAzyme. This sensor effectively enhanced the electrical signal through the synergistic effect of Cu-TCPP and the DNAzyme. It exhibited good electrochemical sensing performance in the linear range of 0.08 μM to 0.11 mM with a LOD of 0.03 μM. Although many nanozymes have been developed, it is challenging to develop single-component nanozymes with comprehensive multi-enzyme-like activities. Yang et al.113 synthesized FePCN nano MOFs with various catalytic properties. FePCN demonstrated high POD-like and OXD-like activities, successfully achieving highly sensitive H2O2 detection under neutral conditions, with a linear range of 5–200 μM and a LOD of 0.48 μM (Fig. 9D).
3.2 Analytes affect the active site of MOF nanozymes
The active sites of MOF nanozymes can catalyze reactions of analyte substances to generate detectable signal molecules, thereby enabling sensitive detection of the analyte substances. The interaction between analyte substances and active sites may cause changes in the catalytic performance and surface properties of MOF nanozymes, offering new insights for designing sensors based on the regulation of MOF nanozyme activity. Currently, sensing mechanisms based on specific interactions between analytes and active sites are mainly divided into two categories: one where the analyte substance directly affects the active sites of the nanozyme, and another where the analyte substance indirectly affects the active sites of the MOF nanozymes.3.2.1.1 Inhibit catalytic activity. Some analyte substances can influence the catalytic activity of MOF nanozymes through interactions with active sites. For instance, when external substances such as inorganic anions18 or metal cations173 are introduced, these molecules can weaken or inhibit the catalytic activity of MOF nanozymes, thereby achieving a signal-off detection response.
Protein phosphorylation plays a key role in cell signaling and is a widespread biological regulatory mechanism essential for controlling protein function. Many human diseases are associated with abnormal phosphorylation, and in-depth study of protein phosphorylation status is critically important for biomedical and clinical applications. In this regard, Wang et al.121 used a Zr-MOF with POD-like activity to identify and quantify phosphorylated proteins. In this process, phosphorylated proteins are anchored to the surface of the MOF nanozymes through strong interactions with Zr nodes (Fig. 10A), leading to the inhibition of the Zr-MOF nanozyme's activity, thereby enabling simple quantification with a LOD of 0.16 mg mL−1. By simultaneously monitoring two independent and opposing reactions generated by the analyte, signal amplification can be achieved, resulting in higher sensitivity in detection. Li et al.174 designed a UiO-66(Fe/Zr)-NH2 trifunctional nanozyme for detecting Pi (Fig. 10B). This MOFs exhibits strong fluorescence at 435 nm through a fluorescent organic ligand and catalyzes the substrate OPD via Fe3+/Fe2+ nodes to generate the fluorescence-enhanced OPDox (555 nm). OPDox quenches the original fluorescence of UiO-66, while the presence of Pi specifically adsorbs to Zr4+ nodes, reducing the nanozyme's activity, decreasing OPDox production, restoring 435 nm fluorescence, and inversely decreasing the 555 nm signal. This mechanism enables highly sensitive detection of Pi, with a LOD of 85 nM and a linear range of 0.2–266.7 μM.
 | ||
| Fig. 10 (A) illustrates the inhibition of the Zr-MOF peroxidase-like activity by phosphorylated proteins. (Reproduced from ref. 121.) (B) illustration of the ratiometric fluorescent Pi sensing platform based on UiO-66(Fe/Zr)-NH2 with three functions of intrinsic fluorescence, peroxidase-mimetic activity, and specific recognition towards Pi. (Reproduced from ref. 174.) (C) Illustration of the dual-channel ratiometric colorimetric sensing of Pi based on the analyte-induced differential oxidase-like activity changes of oxidized UiO-66(Ce/Zr). (Reproduced from ref. 176.) (D) Flow chart for detecting H2O2, Cys and Hg2+. (Reproduced from ref. 168.) (E) Schematic of the inner-filter effect between NH2-MIL-101 and DAP in the NH2-MIL-101/OPD/H2O2 system, and ACP sensing via PPi-mediated FL tuning of the NH2-MIL-101/OPD/H2O2 system. (Reproduced from ref. 181.) (F) Schematic representation of the MOF-MOF cascade catalytic and dual-mode sensors. (Reproduced from ref. 184.) | ||
The interaction between the analyte and the ligands of MOF nanozymes can alter the conformation and charge distribution of the ligands, thereby affecting the coordination environment of metal ions and their catalytic performance. Wang et al.175 designed and prepared a MOFs nanozyme (Tb-OBBA-Hemin) with dual catalytic and luminescent functions for the detection and degradation of 17β-estradiol (E2) and its derivatives. This MOFs nanozyme is composed of luminescent Tb3+ ions, hemin, and a light-harvesting ligand, and it not only mimics HRP in degrading E2 but also enables ultrasensitive detection of E2 through luminescent signals, with a LOD of 50 pM.
3.2.1.2 Change in surface properties. Nanozyme catalysis is a heterogeneous surface reaction process and changes in surface properties can have an impact on its activity. Certain substances can interact with nanozymes through precipitation, adsorption, and coordination, altering the surface chemical properties of the nanozymes and thereby influencing their enzyme-like characteristics. This can serve as an effective strategy for developing efficient MOF nanozymes sensors.
Phosphate ions (Pi) is an important indicator for water quality monitoring. Li et al.176 presented a dual-channel colorimetric sensing method based on UiO-66(Ce/Zr) (Fig. 10C). This method regulates the charge by adsorbing Pi onto the positively charged UiO-66(Ce/Zr), promoting TMB oxidation and inhibiting ABTS oxidation. Compared to traditional single-signal sensors, this method offers a wider linear detection range (3.3–666.7 μM) and a lower detection limit (1.1 μmol L−1). If sludge and wastewater containing heavy metal ions are used as fertilizer or for irrigation, it can lead to soil contamination, causing heavy metal ion accumulation in crops, which can severely harm human health through the food chain. Hexavalent chromium (Cr(VI)) is highly toxic and highly mobile, making it easily spread and bioaccumulate in the environment. Therefore, developing sensitive and rapid methods for detecting Cr(VI) is of urgent importance. Shi et al.177 prepared MOF-199 with POD-like activity. By adsorbing Cr(VI) onto its surface, a negatively charged MOF-199@Cr(VI) is formed, accelerating the adsorption of positively charged TMB molecules on the surface of MOF-199@Cr(VI). MOF-199 facilitates the electron transfer process between Cr(VI) and neighboring TMB. Finally, Cr(VI) is reduced to Cr(III), and TMB is oxidized to blue oxTMB. Based on this, a convenient and cost-effective colorimetric sensor was developed. This method shows a good linear relationship for the detection of hexavalent chromium, with a linear range of 0.1–30 μM and a LOD of 20 nM. The metal in metal oxides can sacrifice itself to provide metal ions, thereby initiating the growth of MOFs. The nucleation and growth of MOFs on metal oxide templates can enhance their catalytic activity and improve sensor sensitivity. Wang et al.178 constructed a CeO2NRs-MOF-based colorimetric sensing platform aimed at efficient detection of hexavalent chromium. By growing MOF in situ on the surface of CeO2NRs, this platform significantly enhanced its OXD-like activity. In the presence of trace amounts of Cr(VI), CeO2NRs-MOF can accelerate the oxidation of TMB, with a linear range of 0.03–5 μM. On the other hand, as another heavy metal pollutant, Ag+ is highly toxic to the nervous system, liver, and kidneys. Li et al.179 synthesized a noble metal-free ZIF-8/GO probe for colorimetric detection of Ag+ in water and human serum. This method relies on the POD-like activity of ZIF-8/GO triggered by Ag+ to oxidize TMB, accompanied by an increase in oxTMB absorption intensity, with a LOD of 1.43 nM.
3.2.2.1 Masking/exposing the active site. Catalytic reactions primarily occur at the interface of catalysts, so the exposure or masking of active sites becomes a key factor, which can influence the catalytic activity of the nanozymes. Analyte molecules can mask or expose the catalytic active sites of MOF nanozymes through mechanisms such as coordination, ion exchange, or precipitation, thereby regulating their catalytic behavior. Generally, the masking of active sites leads to the inhibition of the catalytic activity of MOF nanozymes, while the re-exposure of these active sites helps restore their catalytic function.61
Cu2+ is one of the common pollutants in water. At the same time, Cu2+ is an essential metal element for the human body, and both deficiency and excess of Cu2+ can lead to various diseases. Lv et al.180 developed a colorimetric sensing platform for Cu2+ detection based on the inhibitory effect of Cys on the catalytic activity of ZnO–Co3O4 NCs and its strong binding affinity for Cu2+. In the presence of Cys, the catalytic activity of ZnO–Co3O4 NCs is inhibited, and adding Cu2+ can restore it, leading to the initiation of the signal for Cu2+ detection. The linear range is 2–100 nM, with a detection limit of 1.08 nM. MOF derivatives can retain the original advantages of MOFs while promoting electron transfer, making the material more stable, and they have potential applications in environmental analysis. Lu et al.168 obtained a novel hollow manganese oxide through MOFs@MOFs, which has intrinsic activities mimicking oxidase and peroxidase by adjusting pH. A novel colorimetric sensing platform for detecting Cys and Hg2+ was developed based on the inhibition of colorimetric reaction and OXD-like activity by Cys, and the recovery of OXD-like activity due to the specific complexation of Cys with Hg2+ (Fig. 10D).
3.2.2.2 Cascade catalysis. Acid phosphatase (ACP) is an enzyme that hydrolyzes phosphate esters in acidic media. The qualitative and quantitative evaluation of ACP is clinically important. ACP detection methods include colorimetric and fluorescence assays, with fluorescence detection offering higher sensitivity. Li et al.181 detected ACP using a NH2-MIL-101/OPD/H2O2 system mediated by pyrophosphate ions (PPi). The specific binding of PPi to the iron center of NH2-MIL-101 inhibits catalytic activity, causing fluorescence quenching. The addition of ACP leads to PPi hydrolysis, restoring fluorescence (Fig. 10E), with a LOD of 0.005 U L−1. Acetylcholinesterase (AChE) is the predominant cholinesterase in the body. It ensures proper transmission of nerve signals. Chen et al.182 utilized Co3O4@Co–Fe oxide double-shelled nanocages (DSNCs) with POD-like activity, combined with an AChE/ATCh cascade enzymatic reaction system. Based on this, they developed an ultra-sensitive colorimetric platform for detecting AChE. Organophosphates (OPs) irreversibly inhibit AChE, making it possible to selectively detect OPs by monitoring AChE activity in environmental samples. Luo et al.183 introduced manganese ions into Fe-MIL(53), imparting choline degradation properties to the material. OPs effectively inhibit this process by suppressing AChE activity. Based on this, a simple and sensitive cascade reaction system was developed for the colorimetric identification of OPs, capable of detecting methyl parathion and chlorpyrifos in the ranges of 10–120 nM and 5–50 nM, respectively.
Our group proposed and constructed a MOF-on-MOF dual nano-enzymatic cascade system (MOF-818@PMOF(Fe)) with enhanced catalytic effect and successfully used it for colorimetric/chemiluminescent (CL) bimodal aptamer sensing (Fig. 10F).184 MOF-818 has catechol-like oxidase activity, which can catalyse the oxidation of the substrate and produce the intermediate H2O2. By using in situ-generated H2O2, the On the one hand, it can effectively avoid the addition of exogenous H2O2, which causes excessive local molecular concentration and damage to the material, and on the other hand, it can avoid the loss of H2O2 due to its own degradation. Another MOFs, PMOF(Fe), has POD-like activity, which catalyzes the substrate TMB chromogenic or luminescence to achieve colorimetric/chemiluminescence signal amplification by using the proximity effect of the generated H2O2 to the active site of PMOF(Fe) at the atomic scale and the spatially restricted domains of the MOFs pores and other sensitization mechanisms. In addition, the combination of nucleic acid aptamer sensing strategy has achieved highly sensitive and specific detection of organophosphorus pesticides (OPs).
3.3 Dual-site cascade catalysis by MOF nanozymes
A dual-active-site cascade catalytic system refers to a mechanism where two different active sites sequentially participate in two or more consecutive reaction steps. In this system, the first active site catalyzes the formation of an intermediate product, which is then captured by the second active site for further catalysis in the subsequent reaction step.3.4 Analytes inhibit the catalytic reaction of MOF nanozymes
Besides directly affecting nanozyme activity, many external species also influence the catalytic reaction system of nanozymes. Some reducing substances, such as biothiols and dopamine (DA), can inhibit or hinder the nanozyme-catalyzed TMB chromogenic reaction. On one hand, these reducing substances can competitively oxidize with the TMB substrate. On the other hand, they can reduce oxTMB back to TMB. These reducing substances inhibit the TMB chromogenic reaction. Utilizing this principle, the nanozyme-catalyzed reaction system can be widely used to detect these reducing substances. | ||
| Fig. 12 (A) Schematic of peroxidase-like activity of [Cu(PDA)(DMF)] and its application for colorimetric DA detection. (Reproduced from ref. 194.) (B) Reaction mechanism for ascorbic acid (AA) detection bioassay. (Reproduced from ref. 195.) | ||
Ascorbic acid (AA) plays an important regulatory role in metabolic reactions in the human body. AA deficiency can lead to scurvy, while an excess of AA can cause kidney stones, diarrhea, stomach cramps, etc. Accurate quantification of AA is crucial for disease diagnosis. AA can reduce oxTMB to form colorless TMB, resulting in a fading color change. Therefore, most AA detection methods are based on colorimetric sensing. Wu et al.194 developed a highly sensitive colorimetric platform for AA detection using FeCoNPs@PNC with OXD-like activity. The mechanism of this platform involves FeCoNPs@PNC catalyzing the oxidation of TMB to produce the blue oxidized product oxTMB. AA, as a reducing agent, reacts with oxTMB, reducing it to a colorless or light-colored state, causing the blue color of the solution to fade. This study demonstrates a new hybrid material based on MOF derivatives to replace expensive natural enzymes and noble metal nanozymes. Additionally, single-atom nanozymes, due to their high activity and atomic utilization, provide a novel signaling element for the development of various biosensing technologies. Similarly, Cheng et al.195 established a paper-based biosensor for AA detection using CNT/FeNC SAN with POD-like activity. This paper-based biosensor produces reliable signals at AA concentrations ranging from 0.1 to 0.1–10 × 10−6 M, with a LOD of 0.03 × 10−6 M (Fig. 12B).
3.5 Signal labelling based on MOF nanozymes
MOFs nanozymes were initially designed as substitutes for natural enzymes. Therefore, MOF nanozymes can replace natural enzymes to amplify detection signals while maintaining good stability and low cost. Typically, MOF nanozymes can serve as an ideal substitute for natural enzymes in enzyme-linked immunosorbent assays (ELISA). Not only can it replace traditional natural enzymes to achieve similar signal amplification effects, but its high stability and lower production costs also reduce the overall cost of ELISA detection, while improving the reliability and cost-effectiveness of the detection process. | ||
| Fig. 13 (A) Changes in the bioactivities of MOFs@Ab2 (black) and HRP@Ab2 (red) under different conditions. (Reproduced from ref. 198.) (B) Schematic diagram of the dual-mode immunosensor by using Co/NCNT as oxidase-mimic. (Reproduced from ref. 199.) (C) (a) Schematic illustration of the colorimetric biosensor to detect S. typhimurium (b) UV-Vis platform of catalytic activity of ZrPr-MOFs in the presence of different concentrations of S. typhimurium. (Reproduced from ref. 201.) | ||
4. Conclusions
This paper reviews the latest research progress in the design and functionalization of catalytic active sites of MOF nanozymes, as well as their applications in the field of analytical sensing. The strategies for designing active sites are discussed in detail, including the selection of metal centers, functionalization of ligands, and adjustment of pore structures to optimize substrate diffusion and reaction. In terms of sensing analysis, the paper reviews some groundbreaking advances in the application of MOF nanozymes in sensors, where the sensing mechanism typically involves using MOF nanozymes as active components of sensors. Their high selectivity and sensitivity towards specific analytes enable the detection of analyte substances, including the quantitative analysis of biomolecules and environmental pollutants. With a deeper understanding of the structure–performance relationship of MOF nanozymes and continuous optimization of synthesis methods, their potential roles in various fields will see greater development. However, to achieve this goal, the following challenges must be overcome:(1) In the process of using MOFs to mimic natural enzymes, the majority of the mimic enzyme activity relies on the structural characteristics of their metal centers, while examples of enzyme activity based on active ligands are relatively few. In view of this, developing a diverse range of organic ligands and combining these ligands with catalytically active metal ions has become an urgently needed area of exploration. This strategy is expected not only to diversify enzyme-like activities but also to further expand the application scope of MOFs in the field of catalysis.
(2) MOF derivatives may contain partially amorphous or unevenly crystallized regions, making their structures difficult to precisely define. Therefore, it is crucial to develop more precise synthesis strategies and characterization techniques to reveal the exact structure of MOF derivatives, including amorphous regions, pore distribution, and dopant distribution, while enhancing the predictability of the structure–performance relationship. Based on an in-depth understanding of precise structures, it will become possible to accurately control the synthesis of high-performance MOF materials with high catalytic activity, excellent stability, and good dispersibility.
(3) MOF nanozymes may affect the performance of biomolecules such as aptamers. For example, the surface properties of MOF nanozymes could influence the three-dimensional structure of aptamers. Currently, there are few reports on the performance changes of these biomolecules induced by MOF nanozymes. By carefully designing the chemical properties and morphology of MOFs, their interactions can be optimized, which could enhance their potential applications in biosensing and bio diagnostics.
(4) Currently, integrating advanced sensing technologies with portable devices (such as smartphones) to create handheld instruments for on-site analysis is an emerging field in point-of-care testing (POCT). Within this framework, detection technologies using MOF nanozymes, low-cost test strips, and smartphone-based devices are expected to attract increasing research interest. This exploration aims to develop novel, efficient, and cost-effective sensing platforms to meet the needs of rapid, on-site analysis and play a significant role in fields such as medical diagnostics and environmental monitoring.
(5) Despite the numerous advantages of MOF nanozymes, most MOF materials exhibit relatively poor water and chemical stability, limiting their structural integrity under harsh conditions. This significantly constrains their performance in long-term continuous monitoring applications. Practical environments often involve exposure to water, acids, alkalis, or coordination anions, which destabilize MOFs and hinder their practical utility. Therefore, constructing chemically stable MOF nanozymes is of great importance. The stability of MOFs primarily depends on their framework structure, specifically the metal–ligand bond strength. This can be improved by selecting appropriate metal ions or coordination atoms to enhance bond strength. Additionally, introducing hydrophobic groups on bridging ligands reduces the hydrophilicity of MOF pores, substantially increasing their stability in aqueous environments. Other strategies to enhance chemical stability include increasing ligand rigidity, enhancing network interpenetration, and employing other structural modifications. Another critical challenge is the reusability of MOF nanozymes, which limits their application scope. Most MOF nanozymes in current sensing systems lack recyclability, leading to inefficiencies and potential environmental impact. Improving their recyclability could reduce enzyme consumption and minimize environmental pollution, promoting green chemistry and sustainable development. This approach aligns with global sustainability initiatives while driving sensing technologies toward higher efficiency and environmental friendliness.
(6) MOF nanozymes demonstrate high sensitivity, reliability, and cost-effectiveness in the field of in vitro diagnostics due to their catalytic signal amplification properties. For instance, they can catalyze the oxidation or reduction of substrates, enhance immune responses, and produce detectable signals for rapid disease diagnosis. Moreover, their potential for clinical translation is steadily advancing. Recently, MOF nanozymes have shown exceptional promise in regulating biological processes, making significant strides in applied research for in vivo diagnostics and opening new avenues for disease treatment. However, the toxicity of MOF nanozymes is a critical concern when applied to living organisms. Extensive studies have investigated their potential toxicity, emphasizing the need for comprehensive risk assessments to facilitate clinical application. This is particularly crucial for in vivo monitoring and bioimaging, where toxicity must be carefully evaluated. A promising approach involves using low-toxicity or non-toxic elements in their synthesis. Additionally, developing safer MOF nanozymes through modifications in shape, surface chemistry, and size offers viable strategies. Enhancing their recyclability, degradability, and excretion can also mitigate toxicity concerns. These optimization measures will further support the clinical translation of MOF nanozymes, enabling them to play a more significant role in disease diagnosis and treatment.
(7) In recent years, machine learning (ML), as a data-driven intelligent analysis tool, has made significant progress in various fields, offering new ideas and approaches for solving complex problems. The performance of MOF nanozymes in catalytic reactions is influenced by various factors, such as structural design, the choice of metal centers, and the properties of ligands. Traditional experimental methods often require numerous trials and optimizations, leading to low efficiency and high costs. With the development of machine learning technology, data-driven models can uncover underlying patterns in complex multidimensional data, predict the performance of MOF nanozymes, and provide scientific guidance for catalyst design. In studies combining MOF nanozymes and machine learning, machine learning algorithms can process large amounts of experimental data, identify key factors affecting catalytic performance, optimize MOF design parameters, and predict the performance of novel MOF nanozymes. By integrating the predictive capabilities of machine learning with the advanced material properties of MOFs, the development process of MOF nanozymes can be accelerated, improving catalytic efficiency and selectivity. Additionally, the integration of biochemical sensors with artificial intelligence (AI) has significantly enhanced analytical performance. Cutting-edge AI technologies, particularly the combination of machine learning and deep learning (DL) algorithms, have enabled the analysis of vast sensor data and the identification of complex biomolecular interactions, greatly advancing biochemical sensing technologies.
In summary, although MOF nanozymes show great promise for future applications, fully realizing their immense potential across various fields hinges on addressing the aforementioned challenges. As a crucial part of the functional materials field, future research on MOF nanozymes will not only deepen our understanding of their scientific nature but also enable translational applications in various domains. Promoting interdisciplinary collaboration can maximize the application potential of MOF nanozymes, meeting the diverse needs of societal development. Actively addressing challenges and fostering interdisciplinary collaboration will enable MOF nanozymes to play an increasingly critical role in catalytic science and analytical sensing technologies, leading to revolutionary innovations and advancements in environmental monitoring, disease diagnosis, and biosensing, thus making significant contributions to the sustainable development of human society.
Author contributions
Author contributions are specified based on the CRediT contributorship model. Ziyan Zhang: original draft, investigation, conceptualization. Yujie Li: original draft, investigation, conceptualization. Zhishuang Yuan: investigation. Lingxia Wu: supervision, funding acquisition. Jiping Ma: supervision, resources. Weiqiang Tan: supervision, resources. Yingjie Sun: supervision, resources. Guangyao Zhang: conceptualization, supervision, funding acquisition. Huining Chai: conceptualization, supervision, writing-review & editing, project administration, funding acquisition.Data availability
Data availability is not applicable to this article as no new data were created or analyzed in this study.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 22204089, 21801158 and 22306105), Natural Science Foundation of Shandong Province (No. ZR2023MB016 and ZR2022QB135).References
- D. Jiang, D. Ni, Z. T. Rosenkrans, P. Huang, X. Yan and W. Cai, Chem. Soc. Rev., 2019, 48, 3683–3704 RSC.
- Q. Wang, H. Wei, Z. Zhang, E. Wang and S. Dong, TrAC, Trends Anal. Chem., 2018, 105, 218–224 CrossRef CAS.
- J. Wu, X. Wang, Q. Wang, Z. Lou, S. Li, Y. Zhu, L. Qin and H. Wei, Chem. Soc. Rev., 2019, 48, 1004–1076 RSC.
- P. Geng, L. Wang, M. Du, Y. Bai, W. Li, Y. Liu, S. Chen, P. Braunstein, Q. Xu and H. Pang, Adv. Mater., 2022, 34, 2107836 CrossRef CAS PubMed.
- M. Du, P. Geng, C. Pei, X. Jiang, Y. Shan, W. Hu, L. Ni and H. Pang, Angew. Chem., Int. Ed., 2022, 61, e202209350 CrossRef CAS PubMed.
- G. Zhang, Y. Lu, Y. Yang, H. Yang, Z. Yang, S. Wang, W. Li, Y. Sun, J. Huang, Y. Luo, H.-Y. Chen, Y.-F. Liao, H. Ishii, S. Gull, M. Shakouri, H.-G. Xue, Y. Hu and H. Pang, J. Am. Chem. Soc., 2024, 146, 16659–16669 CrossRef CAS PubMed.
- H. Zhao, X. Tan, H. Chai, L. Hu, H. Li, L. Qu, X. Zhang and G. Zhang, Chin. Chem. Lett., 2024, 110571, DOI:10.1016/j.cclet.2024.110571.
- S. J. Lee and S. G. Telfer, Angew. Chem., Int. Ed., 2023, 62, e202306341 CrossRef CAS PubMed.
- H. Jiang, Q. Gong, R. Zhang and H. Yuan, Coord. Chem. Rev., 2024, 499, 215501 CrossRef CAS.
- G. Fang, S.-X. Bao, G.-X. Zhou and C.-C. Ge, Rare Met., 2024, 43, 900–914 CrossRef CAS.
- X. Huang, S. Zhang, Y. Tang, X. Zhang, Y. Bai and H. Pang, Coord. Chem. Rev., 2021, 449, 214216 CrossRef CAS.
- F. Wang, L. Chen, D. Liu, W. Ma, P. Dramou and H. He, TrAC, Trends Anal. Chem., 2020, 133, 116080 CrossRef CAS.
- Y. Li, H. Chai, Z. Yuan, C. Huang, S. Wang, Y. Sun, X. Zhang and G. Zhang, Chem. Eng. J., 2024, 496, 153884 CrossRef CAS.
- S. Wang, C. M. McGuirk, A. d’Aquino, J. A. Mason and C. A. Mirkin, Adv. Mater., 2018, 30, 1800202 CrossRef PubMed.
- H. Xu, P. Geng, W. Feng, M. Du, D. J. Kang and H. Pang, Nano Res., 2024, 17, 3472–3492 CrossRef CAS.
- Z. Zhang, Z. Yuan, Z. Zhang, J. Guan, W. Tan, G. Zhang and H. Chai, Appl. Organomet. Chem., 2024, e7838, DOI:10.1002/aoc.7838.
- L. Gao, J. Zhuang, L. Nie, J. Zhang, Y. Zhang, N. Gu, T. Wang, J. Feng, D. Yang, S. Perrett and X. Yan, Nat. Nanotechnol., 2007, 2, 577–583 CrossRef CAS PubMed.
- D. Hou, Y. You, X. Wu, C. Li, S. Wu, C. Zhang and Y. Xian, Sens. Actuators, B, 2021, 332, 129508 CrossRef CAS.
- W. Dong, X. Liu, W. Shi and Y. Huang, RSC Adv., 2015, 5, 17451–17457 RSC.
- Z. Hu, Y. Yin, Q. Liu and X. Zheng, Analyst, 2019, 144, 2716–2724 RSC.
- Y. L. Liu, X. J. Zhao, X. X. Yang and Y. F. Li, Analyst, 2013, 138, 4526–4531 RSC.
- Y. Xia, K. Sun, Y.-N. Zuo, S. Zhu and X.-E. Zhao, Chin. Chem. Lett., 2022, 33, 2081–2085 CrossRef CAS.
- J. W. Zhang, H. T. Zhang, Z. Y. Du, X. Wang, S. H. Yu and H. L. Jiang, Chem. Commun., 2014, 50, 1092–1094 RSC.
- T. Lin, Y. Qin, Y. Huang, R. Yang, L. Hou, F. Ye and S. Zhao, Chem. Commun., 2018, 54, 1762–1765 RSC.
- C. Gao, H. Zhu, J. Chen and H. Qiu, Chin. Chem. Lett., 2017, 28, 1006–1012 CrossRef CAS.
- L. Ai, L. Li, C. Zhang, J. Fu and J. Jiang, Chemistry, 2013, 19, 15105–15108 CrossRef CAS PubMed.
- J. He, Y. Zhang, X. Zhang and Y. Huang, Sci. Rep., 2018, 8, 5159 CrossRef PubMed.
- D. Chen, B. Li, L. Jiang, D. Duan, Y. Li, J. Wang, J. He and Y. Zeng, RSC Adv., 2015, 5, 97910–97917 RSC.
- J. Lu, Y. Xiong, C. Liao and F. Ye, Anal. Methods, 2015, 7, 9894–9899 RSC.
- W. Xie, M. Tian, X. Luo, Y. Jiang, N. He, X. Liao and Y. Liu, Sens. Actuators, B, 2020, 302, 127180 CrossRef CAS.
- Y. Jiang, Q. M. Yang, Q. J. Xu, S. Y. Lu, L. Y. Hu, M. W. Xu and Y. S. Liu, Anal. Biochem., 2019, 577, 82–88 CrossRef CAS PubMed.
- J. Wu, Z. Wang, X. Jin, S. Zhang, T. Li, Y. Zhang, H. Xing, Y. Yu, H. Zhang, X. Gao and H. Wei, Adv. Mater., 2021, 33, 2005024 CrossRef CAS PubMed.
- X. Wu, J. Ge, C. Yang, M. Hou and Z. Liu, Chem. Commun., 2015, 51, 13408–13411 RSC.
- Z. Neisi, Z. Ansari-Asl, S. Jafarinejad-Farsangi, M. E. Tarzi, T. Sedaghat and V. Nobakht, Colloids Surf., B, 2019, 178, 365–376 CrossRef CAS PubMed.
- Q. Jin, W. Zhu, D. Jiang, R. Zhang, C. J. Kutyreff, J. W. Engle, P. Huang, W. Cai, Z. Liu and L. Cheng, Nanoscale, 2017, 9, 12609–12617 RSC.
- J. He, J. Dong, Y. Hu, G. Li and Y. Hu, Nanoscale, 2019, 11, 6089–6100 RSC.
- S. Patra, S. Sene, C. Mousty, C. Serre, A. Chausse, L. Legrand and N. Steunou, ACS Appl. Mater. Interfaces, 2016, 8, 20012–20022 CrossRef CAS PubMed.
- S. Shams, W. Ahmad, A. H. Memon, Y. Wei, Q. Yuan and H. Liang, RSC Adv., 2019, 9, 40845–40854 RSC.
- B. Wang, P. Liu, Y. Hu, H. Zhao, L. Zheng and Q. Cao, Dalton Trans., 2023, 52, 2309–2316 RSC.
- Z. Li, Int. J. Electrochem. Sci., 2020, 15, 7423–7433 CrossRef.
- Y. Yang, J. Xu, Y. Guo, X. Wang, L.-P. Xiao and J. Zhou, ACS Sustainable Chem. Eng., 2022, 10, 5489–5499 CrossRef CAS.
- D. Sun, L. Chen, L. Zeng, X. Shi and J. Lu, J. Mater. Chem. A, 2023, 11, 31–40 RSC.
- H. Liang, F. Lin, Z. Zhang, B. Liu, S. Jiang, Q. Yuan and J. Liu, ACS Appl. Mater. Interfaces, 2017, 9, 1352–1360 CrossRef CAS PubMed.
- J. Wang, R. Huang, W. Qi, R. Su and Z. He, Chem. Eng. J., 2022, 434, 134677 CrossRef CAS.
- M. Li, J. Chen, W. Wu, Y. Fang and S. Dong, J. Am. Chem. Soc., 2020, 142, 15569–15574 CrossRef CAS PubMed.
- K. Yu, M. Li, H. Chai, Q. Liu, X. Hai, M. Tian, L. Qu, T. Xu, G. Zhang and X. Zhang, Chem. Eng. J., 2023, 451, 138321 CrossRef CAS.
- B. Li, D. Chen, J. Wang, Z. Yan, L. Jiang, D. Deliang, J. He, Z. Luo, J. Zhang and F. Yuan, Sci. Rep., 2014, 4, 6759 CrossRef PubMed.
- C. Wang, J. Gao, Y. Cao and H. Tan, Anal. Chim. Acta, 2018, 1004, 74–81 CrossRef CAS PubMed.
- Z. Qi, L. Wang, Q. You and Y. Chen, Biosens. Bioelectron., 2017, 96, 227–232 CrossRef CAS PubMed.
- H. Yang, J. Liu, X. Feng, F. Nie and G. Yang, Anal. Bioanal. Chem., 2021, 413, 4407–4416 CrossRef CAS PubMed.
- X.-E. Zhao, Y.-N. Zuo, Y. Xia, J. Sun, S. Zhu and G. Xu, Sens. Actuators, B, 2022, 371, 132548 CrossRef CAS.
- H. Tan, Q. Li, Z. Zhou, C. Ma, Y. Song, F. Xu and L. Wang, Anal. Chim. Acta, 2015, 856, 90–95 CrossRef CAS PubMed.
- M. Y. Shi, M. Xu and Z. Y. Gu, Anal. Chim. Acta, 2019, 1079, 164–170 CrossRef CAS PubMed.
- M. Ying, G. Yang, Y. Xu, H. Ye, X. Lin, Y. Lu, H. Pan, Y. Bai and M. Du, Analyst, 2021, 147, 40–47 RSC.
- J. Jacobsen, A. Ienco, R. D'Amato, F. Costantino and N. Stock, Dalton Trans., 2020, 49, 16551–16586 RSC.
- F. A. Son, A. Atilgan, K. B. Idrees, T. Islamoglu and O. K. Farha, Inorg. Chem. Front., 2020, 7, 984–990 RSC.
- D. Chao, Q. Dong, Z. Yu, D. Qi, M. Li, L. Xu, L. Liu, Y. Fang and S. Dong, J. Am. Chem. Soc., 2022, 144, 23438–23447 CrossRef CAS PubMed.
- Y. Liu, H. Li, W. Liu, J. Guo, H. Yang, H. Tang, M. Tian, H. Nie, X. Zhang and W. Long, ACS Appl. Mater. Interfaces, 2022, 14, 54587–54597 CrossRef CAS PubMed.
- R. Dalapati, B. Sakthivel, M. K. Ghosalya, A. Dhakshinamoorthy and S. Biswas, CrystEngComm, 2017, 19, 5915–5925 RSC.
- Y. Zhang, X. Zeng, X. Jiang, H. Chen and Z. Long, Microchem. J., 2019, 149, 103967 CrossRef CAS.
- C. Wang, G. Tang and H. Tan, Microchim. Acta, 2018, 185, 475 CrossRef PubMed.
- Y. Xiong, S. Chen, F. Ye, L. Su, C. Zhang, S. Shen and S. Zhao, Chem. Commun., 2015, 51, 4635–4638 RSC.
- H. N. Abdelhamid and W. Sharmoukh, Microchem. J., 2021, 163, 105873 CrossRef CAS.
- R. Gao, N. Ye, X. Kou, Y. Shen, H. Yang, T. Wu, S. Huang, G. Chen and G. Ouyang, Chem. Commun., 2022, 58, 12720–12723 RSC.
- S. Rojas-Buzo, P. Concepcion, J. L. Olloqui-Sariego, M. Moliner and A. Corma, ACS Appl. Mater. Interfaces, 2021, 13, 31021–31030 CrossRef CAS PubMed.
- L. Luo, L. Huang, X. Liu, W. Zhang, X. Yao, L. Dou, X. Zhang, Y. Nian, J. Sun and J. Wang, Inorg. Chem., 2019, 58, 11382–11388 CrossRef CAS PubMed.
- X. Zhang, D. Wu, Y. Wu and G. Li, Biosens. Bioelectron., 2021, 172, 112776 CrossRef CAS PubMed.
- T. D. Tran, P. T. Nguyen, T. N. Le and M. I. Kim, Biosens. Bioelectron., 2021, 182, 113187 CrossRef CAS PubMed.
- C. Wang, J. Fei, K. Wang and J. Li, Angew. Chem., Int. Ed., 2020, 59, 18960–18963 CrossRef CAS PubMed.
- S. Rashtbari and G. Dehghan, J. Hazard. Mater., 2021, 406, 124340 CrossRef CAS PubMed.
- S. Liang, X.-L. Wu, J. Xiong, X. Yuan, S.-L. Liu, M.-H. Zong and W.-Y. Lou, Chem. Eng. J., 2022, 450, 138220 CrossRef CAS.
- S. Y. Moon, G. W. Wagner, J. E. Mondloch, G. W. Peterson, J. B. DeCoste, J. T. Hupp and O. K. Farha, Inorg. Chem., 2015, 54, 10829–10833 CrossRef CAS PubMed.
- E. Lopez-Maya, C. Montoro, L. M. Rodriguez-Albelo, S. D. Aznar Cervantes, A. A. Lozano-Perez, J. L. Cenis, E. Barea and J. A. Navarro, Angew. Chem., Int. Ed., 2015, 54, 6790–6794 CrossRef CAS PubMed.
- J. E. Mondloch, M. J. Katz, W. C. Isley 3rd, P. Ghosh, P. Liao, W. Bury, G. W. Wagner, M. G. Hall, J. B. DeCoste, G. W. Peterson, R. Q. Snurr, C. J. Cramer, J. T. Hupp and O. K. Farha, Nat. Mater., 2015, 14, 512–516 CrossRef CAS PubMed.
- S. S. Nadar and V. K. Rathod, Int. J. Biol. Macromol., 2020, 152, 1108–1112 CrossRef PubMed.
- X. Liu, W. Qi, Y. Wang, R. Su and Z. He, Eur. J. Inorg. Chem., 2018, 2018, 4579–4585 CrossRef CAS.
- R. Xu, T. Wu, X. Jiao, D. Chen and C. Li, ACS Appl. Mater. Interfaces, 2023, 15, 30360–30371 CrossRef CAS PubMed.
- M. Xu, L. Feng, L.-N. Yan, S.-S. Meng, S. Yuan, M.-J. He, H. Liang, X.-Y. Chen, H.-Y. Wei, Z.-Y. Gu and H.-C. Zhou, Nanoscale, 2019, 11, 11270–11278 RSC.
- H.-Q. Zheng, C.-Y. Liu, X.-Y. Zeng, J. Chen, J. Lü, R.-G. Lin, R. Cao, Z.-J. Lin and J.-W. Su, Inorg. Chem., 2018, 57, 9096–9104 CrossRef CAS PubMed.
- J. Wang, Y. Zhou, M. Zeng, Y. Zhao, X. Zuo, F. Meng, F. Lv and Y. Lu, Environ. Res., 2022, 203, 111818 CrossRef CAS PubMed.
- W. Yang, L. Zhu and W. Xu, J. Environ. Chem. Eng., 2024, 12, 112358 CrossRef CAS.
- G. Chen, Y. Yu, X. Fu, G. Wang, Z. Wang, X. Wu, J. Ren and Y. Zhao, J. Colloid Interface Sci., 2022, 607, 1382–1390 CrossRef CAS PubMed.
- J. Chen, L. Huang, Q. Wang, W. Wu, H. Zhang, Y. Fang and S. Dong, Nanoscale, 2019, 11, 5960–5966 RSC.
- C. Jin, S. Zhang, Z. Zhang and Y. Chen, Inorg. Chem., 2018, 57, 2169–2174 CrossRef CAS PubMed.
- A. M. Wright, Z. Wu, G. Zhang, J. L. Mancuso, R. J. Comito, R. W. Day, C. H. Hendon, J. T. Miller and M. Dincă, Chem, 2018, 4, 2894–2901 CAS.
- M. Han, M. Ren, Z. Li, L. Qu and L. Yu, New J. Chem., 2022, 46, 10682–10689 RSC.
- G. Ren, F. Dong, Z. Zhao, K. Li and Y. Lin, ACS Appl. Mater. Interfaces, 2021, 13, 52987–52997 CrossRef CAS PubMed.
- J. Rockenberger, E. C. Scher and A. P. Alivisatos, J. Am. Chem. Soc., 1999, 121, 11595–11596 CrossRef CAS.
- Y. Miao, X. Zhao, X. Sun and J. Lv, Food Chem., 2024, 451, 139378 CrossRef CAS PubMed.
- Y. Chen, Q. Tian, H. Wang, R. Ma, R. Han, Y. Wang, H. Ge, Y. Ren, R. Yang, H. Yang, Y. Chen, X. Duan, L. Zhang, J. Gao, L. Gao, X. Yan and Y. Qin, Adv. Mater., 2024, 36, 2206421 CrossRef CAS PubMed.
- J. Dong, H.-D. An, Z.-K. Yue, S.-L. Hou, Y. Chen, Z.-J. Zhang, P. Cheng, Q. Peng and B. Zhao, ACS Cent. Sci., 2021, 7, 831–840 CrossRef CAS PubMed.
- C. Fu, H. Zhou, L. Tan, Z. Huang, Q. Wu, X. Ren, J. Ren and X. Meng, ACS Nano, 2017, 12, 2201–2210 CrossRef PubMed.
- X. Zhao, N. Zhang, T. Yang, D. Liu, X. Jing, D. Wang, Z. Yang, Y. Xie and L. Meng, ACS Appl. Mater. Interfaces, 2021, 13, 36106–36116 CrossRef CAS PubMed.
- K. Zhang, L. Lu, Z. Liu, X. Cao, L. Lv, J. Xia and Z. Wang, Colloids Surf., A, 2022, 650, 129662 CrossRef CAS.
- C. Peng, Y. Xue, X. Zhu, Y. Fan, J. Li and E. Wang, Anal. Chem., 2021, 94, 1465–1473 CrossRef PubMed.
- Y. Wang, M. Zhao, J. Ping, B. Chen, X. Cao, Y. Huang, C. Tan, Q. Ma, S. Wu, Y. Yu, Q. Lu, J. Chen, W. Zhao, Y. Ying and H. Zhang, Adv. Mater., 2016, 28, 4149–4155 CrossRef CAS PubMed.
- X. Qi, H. Tian, X. Dang, Y. Fan, Y. Zhang and H. Zhao, Anal. Methods, 2019, 11, 1111–1124 RSC.
- X. Cheng, X. Zhou, Z. Zheng and Q. Kuang, Chem. Eng. J., 2022, 430, 133079 CrossRef CAS.
- X. Dang and H. Zhao, Talanta, 2020, 210, 120678 CrossRef CAS PubMed.
- S. Li, Y. Hou, Q. Chen, X. Zhang, H. Cao and Y. Huang, ACS Appl. Mater. Interfaces, 2020, 12, 2581–2590 CrossRef CAS PubMed.
- H. Yang, R. Yang, P. Zhang, Y. Qin, T. Chen and F. Ye, Microchim. Acta, 2017, 184, 4629–4635 CrossRef CAS.
- Y. Zhang, C. Dai, W. Liu, Y. Wang, F. Ding, P. Zou, X. Wang, Q. Zhao and H. Rao, Microchim. Acta, 2019, 186, 340 CrossRef PubMed.
- S. Kulandaivel, C. H. Lin and Y. C. Yeh, Chem. Commun., 2022, 58, 569–572 RSC.
- Y. Zhang, Y.-S. Feng, X.-H. Ren, X.-W. He, W.-Y. Li and Y.-K. Zhang, Biosens. Bioelectron., 2022, 196, 113718 CrossRef CAS PubMed.
- Y. Li, G. Zhao, B. An, K. Xu, D. Wu, X. Ren, H. Ma, X. Liu, R. Feng and Q. Wei, Anal. Chem., 2024, 96, 4067–4075 CrossRef CAS PubMed.
- L. Yin, B. Xing, Z. Liu and L. Lu, Chem. Eng. J., 2024, 493, 152663 CrossRef CAS.
- Q. Chen, H. Zhang, H. Sun, Y. Yang, D. Zhang, X. Li, L. Han, G. Wang and Y. Zhang, Food Chem., 2024, 442, 138383 CrossRef CAS PubMed.
- T. Zhang, M. Tang, S. Yang, H. Fa, Y. Wang, D. Huo, C. Hou and M. Yang, Food Chem., 2025, 464, 141780 CrossRef CAS PubMed.
- X. Lai, Y. Shen, S. Gao, Y. Chen, Y. Cui, D. Ning, X. Ji, Z. Liu and L. Wang, Biosens. Bioelectron., 2022, 213, 114446 CrossRef CAS PubMed.
- H. Sun, J. Guan, H. Chai, K. Yu, L. Qu, X. Zhang and G. Zhang, Biosens. Bioelectron., 2024, 251, 116080 CrossRef CAS PubMed.
- Y. Shu, Q. Ye, J. Tan, H. Lv, Z. Liu and Q. Mo, ACS Appl. Nano Mater., 2022, 5, 17909–17918 CrossRef CAS.
- Y. Zhou, B. Zheng, L.-M. Lang, G.-X. Liu and X.-H. Xia, ACS Appl. Nano Mater., 2022, 5, 18761–18769 CrossRef CAS.
- C. Yang, Z. Jiang, Q. Wu, C. Hu, C. Huang, Y. Li and S. Zhen, J. Colloid Interface Sci., 2022, 605, 214–222 CrossRef CAS PubMed.
- H. Cheng, Y. Liu, Y. Hu, Y. Ding, S. Lin, W. Cao, Q. Wang, J. Wu, F. Muhammad, X. Zhao, D. Zhao, Z. Li, H. Xing and H. Wei, Anal. Chem., 2017, 89, 11552–11559 CrossRef CAS PubMed.
- J. Wang, T. Wei, Y. Liu, M. Bao, R. Feng, Y. Qian, X. Yang, L. Si and Z. Dai, Analyst, 2020, 145, 3002–3008 RSC.
- H. Wan, Y. Wang, J. Chen, H. M. Meng and Z. Li, Microchim. Acta, 2021, 188, 130 CrossRef CAS PubMed.
- D. Feng, Z. Y. Gu, J. R. Li, H. L. Jiang, Z. Wei and H. C. Zhou, Angew. Chem., Int. Ed., 2012, 51, 10307–10310 CrossRef CAS PubMed.
- F. Liu, J. He, M. Zeng, J. Hao, Q. Guo, Y. Song and L. Wang, J. Nanopart. Res., 2016, 18, 106 CrossRef.
- C. Q. Sun, Z. L. Huang, L. Liu, M. L. Li and H. Z. Zheng, Anal. Sci., 2018, 34, 933–938 CrossRef CAS PubMed.
- M. Li, L. Liu, Y. Shi, Y. Yang, H. Zheng and Y. Long, New J. Chem., 2017, 41, 7578–7582 RSC.
- L. Wang, Z. Hu, S. Wu, J. Pan, X. Xu and X. Niu, Anal. Chim. Acta, 2020, 1121, 26–34 CrossRef CAS PubMed.
- A. H. Valekar, B. S. Batule, M. I. Kim, K. H. Cho, D. Y. Hong, U. H. Lee, J. S. Chang, H. G. Park and Y. K. Hwang, Biosens. Bioelectron., 2018, 100, 161–168 CrossRef CAS PubMed.
- S. M. Cohen, J. Am. Chem. Soc., 2017, 139, 2855–2863 CrossRef CAS PubMed.
- H. Deng, C. J. Doonan, H. Furukawa, R. B. Ferreira, J. Towne, C. B. Knobler, B. Wang and O. M. Yaghi, Science, 2010, 327, 846–850 CrossRef CAS PubMed.
- F. Cui, Q. Deng and L. Sun, RSC Adv., 2015, 5, 98215–98221 RSC.
- X. Mao, F. He, D. Qiu, S. Wei, R. Luo, Y. Chen, X. Zhang, J. Lei, D. Monchaud, J. L. Mergny, H. Ju and J. Zhou, Anal. Chem., 2022, 94, 7295–7302 CrossRef CAS PubMed.
- W. Zhou, H. Li, B. Xia, W. Ji, S. Ji, W. Zhang, W. Huang, F. Huo and H. Xu, Nano Res., 2018, 11, 5761–5768 CrossRef CAS.
- T. Liu, J. Tian, L. Cui, Q. Liu, L. Wu and X. Zhang, Colloids Surf., B, 2019, 178, 137–145 CrossRef CAS PubMed.
- W. H. Chen, M. Vazquez-Gonzalez, A. Kozell, A. Cecconello and I. Willner, Small, 2018, 14, 1703149 CrossRef PubMed.
- M. Li, Y. Xie, L. Lei, H. Huang and Y. Li, Sens. Actuators, B, 2022, 357, 131429 CrossRef CAS.
- W. Dong, L. Yang and Y. Huang, Talanta, 2017, 167, 359–366 CrossRef CAS PubMed.
- Y. Shu, J. Chen, Z. Xu, D. Jin, Q. Xu and X. Hu, J. Electroanal. Chem., 2019, 845, 137–143 CrossRef CAS.
- Z. Zhao, J. Pang, W. Liu, T. Lin, F. Ye and S. Zhao, Microchim. Acta, 2019, 186, 295 CrossRef PubMed.
- L. Shao, X. Gao, J. Liu, Q. Zheng, Y. Li, P. Yu, M. Wang and L. Mao, ACS Appl. Mater. Interfaces, 2022, 14, 47472–47481 CrossRef CAS PubMed.
- W. Xu, L. Jiao, H. Yan, Y. Wu, L. Chen, W. Gu, D. Du, Y. Lin and C. Zhu, ACS Appl. Mater. Interfaces, 2019, 11, 22096–22101 CrossRef CAS PubMed.
- P. Ling, C. Qian, F. Gao and J. Lei, Chem. Commun., 2018, 54, 11176–11179 RSC.
- X. Zhong, H. Xia, W. Huang, Z. Li and Y. Jiang, Chem. Eng. J., 2020, 381, 122758 CrossRef CAS.
- X. Liu, W. Qi, Y. Wang, D. Lin, X. Yang, R. Su and Z. He, ACS Appl. Mater. Interfaces, 2018, 10, 33407–33415 CrossRef CAS PubMed.
- Z. Zhao, Y. Huang, W. Liu, F. Ye and S. Zhao, ACS Sustainable Chem. Eng., 2020, 8, 4481–4488 CrossRef CAS.
- Q. Wang, X. Zhang, L. Huang, Z. Zhang and S. Dong, Angew. Chem., Int. Ed., 2017, 56, 16082–16085 CrossRef CAS PubMed.
- P. Ling, S. Cheng, N. Chen, C. Qian and F. Gao, ACS Appl. Mater. Interfaces, 2020, 12, 17185–17192 CrossRef CAS PubMed.
- P. George and P. Chowdhury, Chem. Pap., 2023, 77, 1361–1375 CrossRef CAS.
- T. Zhang, Y. Xing, Y. Song, Y. Gu, X. Yan, N. Lu, H. Liu, Z. Xu, H. Xu, Z. Zhang and M. Yang, Anal. Chem., 2019, 91, 10589–10595 CrossRef CAS PubMed.
- Y. Wang, X. Liu, M. Wang, X. Wang, W. Ma and J. Li, Sens. Actuators, B, 2021, 329, 129115 CrossRef CAS.
- X. Li, S. Ding, Z. Lyu, P. Tieu, M. Wang, Z. Feng, X. Pan, Y. Zhou, X. Niu, D. Du, W. Zhu and Y. Lin, Small, 2022, 18, 2203001 CrossRef CAS PubMed.
- M. Lu, C. Wang, Y. Ding, M. Peng, W. Zhang, K. Li, W. Wei and Y. Lin, Chem. Commun., 2019, 55, 14534–14537 RSC.
- Y. Mao, S. Gao, L. Yao, L. Wang, H. Qu, Y. Wu, Y. Chen and L. Zheng, J. Hazard. Mater., 2021, 408, 124898 CrossRef CAS PubMed.
- X. Niu, Q. Shi, W. Zhu, D. Liu, H. Tian, S. Fu, N. Cheng, S. Li, J. N. Smith, D. Du and Y. Lin, Biosens. Bioelectron., 2019, 142, 111495 CrossRef CAS PubMed.
- L. Zhu, H. Zhong, D. Du, T. Li, H. Nguyen, S. P. Beckman, W. Xu, J.-C. Li, N. Cheng and Y. Lin, Nano Res., 2023, 16, 5216–5225 CrossRef CAS.
- Y. Wu, J. Wu, L. Jiao, W. Xu, H. Wang, X. Wei, W. Gu, G. Ren, N. Zhang, Q. Zhang, L. Huang, L. Gu and C. Zhu, Anal. Chem., 2020, 92, 3373–3379 CrossRef CAS PubMed.
- F. Li, N. Li, C. Xue, H. Wang, Q. Chang, H. Liu, J. Yang and S. Hu, Chem. Eng. J., 2020, 382, 122484 CrossRef CAS.
- Y. Wang, A. Cho, G. Jia, X. Cui, J. Shin, I. Nam, K. J. Noh, B. J. Park, R. Huang and J. W. Han, Angew. Chem., Int. Ed., 2023, 62, e202300119 CrossRef CAS PubMed.
- S. Chen, W. Lu, R. Xu, J. Tan and X. Liu, Carbon, 2023, 201, 439–448 CrossRef CAS.
- Y. Zhu, W. Wang, J. Cheng, Y. Qu, Y. Dai, M. Liu, J. Yu, C. Wang, H. Wang, S. Wang, C. Zhao, Y. Wu and Y. Liu, Angew. Chem., Int. Ed., 2021, 60, 9480–9488 CrossRef CAS PubMed.
- Q. Feng, G. Wang, L. Xue, Y. Wang, M. Liu, J. Liu, S. Zhang and W. Hu, ACS Appl. Nano Mater., 2023, 6, 4844–4853 CrossRef CAS.
- W. Wang, Q. Luo, J. Li, L. Li, Y. Li, X. Huo, X. Du, Z. Li and N. Wang, Adv. Funct. Mater., 2022, 32, 2205461 CrossRef CAS.
- Y. Chen, B. Jiang, H. Hao, H. Li, C. Qiu, X. Liang, Q. Qu, Z. Zhang, R. Gao, D. Duan, S. Ji, D. Wang and M. Liang, Angew. Chem., Int. Ed., 2023, e202301879 CAS.
- H. Tan, C. Ma, L. Gao, Q. Li, Y. Song, F. Xu, T. Wang and L. Wang, Chemistry, 2014, 20, 16377–16383 CrossRef CAS PubMed.
- T. Wu, Z. Ma, P. Li, Q. Lu, M. Liu, H. Li, Y. Zhang and S. Yao, Sens. Actuators, B, 2019, 290, 357–363 CrossRef CAS.
- L. Sun, Y. Ding, Y. Jiang and Q. Liu, Sens. Actuators, B, 2017, 239, 848–856 CrossRef CAS.
- Y. Ding, M. Chen, K. Wu, M. Chen, L. Sun, Z. Liu, Z. Shi and Q. Liu, Mater. Sci. Eng., C, 2017, 80, 558–565 CrossRef CAS PubMed.
- Q. Liu, Y. Yang, H. Li, R. Zhu, Q. Shao, S. Yang and J. Xu, Biosens. Bioelectron., 2015, 64, 147–153 CrossRef CAS PubMed.
- H. Wang, W. Fu, Y. Chen, F. Xue and G. Shan, Spectrochim. Acta, Part A, 2021, 246, 119006 CrossRef CAS PubMed.
- Y. Zhuang, X. Zhang, Q. Chen, S. Li, H. Cao and Y. Huang, Mater. Sci. Eng., C, 2019, 94, 858–866 CrossRef CAS PubMed.
- L. Wang, S. Li, X. Zhang and Y. Huang, Talanta, 2020, 216, 121009 CrossRef CAS PubMed.
- W. Dong and Y. Huang, Microchim. Acta, 2020, 187, 11 CrossRef CAS PubMed.
- Y. Cheng, L. Liang, F. Ye and S. Zhao, Biosens., 2021, 11, 204 CrossRef CAS PubMed.
- Z. Lu, Y. Dang, C. Dai, Y. Zhang, P. Zou, H. Du, Y. Zhang, M. Sun, H. Rao and Y. Wang, J. Hazard. Mater., 2021, 403, 123979 CrossRef CAS PubMed.
- Y. Wang, Y. Zhu, A. Binyam, M. Liu, Y. Wu and F. Li, Biosens. Bioelectron., 2016, 86, 432–438 CrossRef CAS PubMed.
- Z. Mu, J. Guo, M. Li, S. Wu, X. Zhang and Y. Wang, Microchim. Acta, 2023, 190, 81 CrossRef CAS PubMed.
- X. Qian Tang, Y. Dan Zhang, Z. Wei Jiang, D. Mei Wang, C. Zhi Huang and Y. Fang Li, Talanta, 2018, 179, 43–50 CrossRef PubMed.
- J. Ma, G. Chen, W. Bai and J. Zheng, ACS Appl. Mater. Interfaces, 2020, 12, 58105–58112 CrossRef CAS PubMed.
- C. Cheng, R. Zhang, J. Wang, Y. Zhang, C. Wen, Y. Tan and M. Yang, Analyst, 2020, 145, 797–804 RSC.
- X. Li, P. Liu, X. Niu, K. Ye, L. Ni, D. Du, J. Pan and Y. Lin, Nanoscale, 2020, 12, 19383–19389 RSC.
- L. Wang and Y. Chen, ACS Appl. Mater. Interfaces, 2020, 12, 8351–8358 CrossRef CAS PubMed.
- X. Li, X. Niu, P. Liu, X. Xu, D. Du and Y. Lin, Sens. Actuators, B, 2020, 321, 128546 CrossRef CAS.
- W. Shi, M. He, W. Li, X. Wei, B. Bui, M. Chen and W. Chen, ACS Appl. Nano Mater., 2021, 4, 802–810 CrossRef CAS.
- Y. Wang, R. P. Liang and J. D. Qiu, Anal. Chem., 2020, 92, 2339–2346 CrossRef CAS PubMed.
- C.-r. Li, J. Hai, L. Fan, S.-l. Li, B.-d. Wang and Z.-y. Yang, Sens. Actuators, B, 2019, 284, 213–219 CrossRef CAS.
- J. Lv, C. Zhang, S. Wang, M. Li and W. Guo, Analyst, 2021, 146, 605–611 RSC.
- S. Li, X. Hu, Q. Chen, X. Zhang, H. Chai and Y. Huang, Biosens. Bioelectron., 2019, 137, 133–139 CrossRef CAS PubMed.
- Q. Chen, X. Zhang, S. Li, J. Tan, C. Xu and Y. Huang, Chem. Eng. J., 2020, 395, 125130 CrossRef CAS.
- L. Luo, Y. Ou, Y. Yang, G. Liu, Q. Liang, X. Ai, S. Yang, Y. Nian, L. Su and J. Wang, J. Hazard. Mater., 2022, 423, 127253 CrossRef CAS PubMed.
- H. Chai, K. Yu, Y. Zhao, Z. Zhang, S. Wang, C. Huang, X. Zhang and G. Zhang, Anal. Chem., 2023, 95, 10785–10794 CrossRef CAS PubMed.
- Q. Zhang, F. Zhang, L. Yu, Q. Kang, Y. Chen and D. Shen, Microchim. Acta, 2020, 187, 244 CrossRef CAS PubMed.
- H. Chai, Y. Li, K. Yu, Z. Yuan, J. Guan, W. Tan, J. Ma, X. Zhang and G. Zhang, Anal. Chem., 2023, 95, 16383–16391 CrossRef CAS PubMed.
- X. Qing, Y. Wang, Y. Zhang, X. Ding, W. Zhong, D. Wang, W. Wang, Q. Liu, K. Liu, M. Li and Z. Lu, ACS Appl. Mater. Interfaces, 2019, 11, 13105–13113 CrossRef CAS PubMed.
- J. Zhao, L. Zhao, C. Lan and S. Zhao, Sens. Actuators, B, 2016, 223, 246–251 CrossRef CAS.
- A. Yildirim and M. Bayindir, Anal. Chem., 2014, 86, 5508–5512 CrossRef CAS PubMed.
- X. D. Xu, H. M. Shi, L. Ma, W. Kang and S. Li, Luminescence, 2011, 26, 93–100 CrossRef CAS PubMed.
- J. Wang, Y. Hu, Q. Zhou, L. Hu, W. Fu and Y. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 44466–44473 CrossRef CAS PubMed.
- J. Wang, W. Li and Y. Q. Zheng, Analyst, 2019, 144, 6041–6047 RSC.
- Y. Liu, M. Zhou, W. Cao, X. Wang, Q. Wang, S. Li and H. Wei, Anal. Chem., 2019, 91, 8170–8175 CrossRef CAS PubMed.
- T. Wu, Z. Ma, P. Li, M. Liu, X. Liu, H. Li, Y. Zhang and S. Yao, Talanta, 2019, 202, 354–361 CrossRef CAS PubMed.
- N. Cheng, J. C. Li, D. Liu, Y. Lin and D. Du, Small, 2019, 15, 1901485 CrossRef CAS PubMed.
- X. Zhang, Y. Li, Q. Chen and Y. Huang, Sens. Actuators, B, 2022, 369, 132365 CrossRef CAS.
- H. Zhang, H. Yang, P. Liu, X. Qin and G. Liu, Talanta, 2022, 237, 122906 CrossRef CAS PubMed.
- Z. Xu, L. L. Long, Y. Q. Chen, M. L. Chen and Y. H. Cheng, Food Chem., 2021, 338, 128039 CrossRef CAS PubMed.
- M. Chen, Z. Liu, Y. Guan, Y. Chen, W. Liu and Y. Liu, Sens. Actuators, B, 2022, 359, 131609 CrossRef CAS.
- W. C. Hu, J. Pang, S. Biswas, K. Wang, C. Wang and X. H. Xia, Anal. Chem., 2021, 93, 8544–8552 CrossRef CAS PubMed.
- E. Mirsadoughi, A. B. Pebdeni and M. Hosseini, Food Control, 2023, 146, 109500 CrossRef CAS.
Footnote |
| † These authors equally contributed to the work. |
| This journal is © the Partner Organisations 2025 |