Open Access Article
Open Access ArticleProduction, labeling, and applications of micro- and nanoplastic reference and test materials†
Guillaume
Crosset-Perrotin‡
 a,
Angélique
Moraz‡
a,
Angélique
Moraz‡
 b,
Raquel
Portela‡
b,
Raquel
Portela‡
 c,
Victor
Alcolea-Rodriguez‡
c,
Victor
Alcolea-Rodriguez‡
 c,
David
Burrueco-Subirà
c,
David
Burrueco-Subirà
 d,
Casey
Smith
d,
Casey
Smith
 e,
Miguel A.
Bañares
e,
Miguel A.
Bañares
 c,
Hosein
Foroutan
c,
Hosein
Foroutan
 f and
D. Howard
Fairbrother
f and
D. Howard
Fairbrother
 *e
*e
aEawag, Swiss Federal Institute of Aquatic Science and Technology, Ueberlandstrasse 133, 8600 Duebendorf, Switzerland
bAgroscope, Swiss Federal Research Station for Agroecology and Agriculture, Reckenholzstrasse 191, 8046 Zürich, Switzerland
cCSIC – Instituto de Catalisis y Petroleoquimica (ICP), C/Marie Curie 2, 28049 Madrid, Spain
dLeitat Technological Center, C/de la Innovació 2, 08225 Terrassa, Barcelona, Spain
eDepartment of Chemistry, Johns Hopkins University, Baltimore, MD, USA. E-mail: howardf@jhu.edu
fDepartment of Civil & Environmental Engineering, Virginia Tech, Blacksburg, VA, USA
First published on 9th April 2025
Abstract
Challenges inherent to the extraction of micro- and nanoplastics (MNPs) from the environment, combined with the limited range of commercially available MNPs, have prompted an increasing number of researchers to generate in-house reference and test MNPs. The first part of this review provides a comprehensive overview of existing MNP production methods, including top-down and bottom-up fabrication techniques. Strengths and weaknesses of different methods are compared and contrasted, and the potential for optimization and control over MNP properties is discussed. Methods to label and to artificially weather MNPs before, during, or after production, as well as appropriate dispersion protocols for introducing MNPs into different media, are also covered. The second part of this review focuses on how reference and test MNPs have been implemented in different types of studies, categorized as toxicity, uptake, fate, and monitoring. Given the wide range of properties needed to fully define MNPs, we propose a set of essential properties that need to be characterized depending on the study type. Looking forward, we suggest future needs, not only in the creation of reference MNPs, but also in experimental protocols that would help to better understand the behavior and impacts of MNPs. Overall, this review aims to provide the necessary information to guide researchers in decision-making regarding which reference MNPs are most appropriate to answer their specific research questions and to serve as a framework that will contribute to obtaining reliable, benchmarked data urgently needed to develop consensus on the fate and risk posed by MNPs.
Environmental significanceThe number of studies on the potential hazard of MNPs is surging, but the lack of representative, traceable and well-characterized reference and test MNPs hampers quality control and inter-study comparability and limits the scope and reliability of the extracted conclusions. This study evaluates the main fabrication methods for such particles, and their respective strengths, weaknesses, scalability, ease of use, cost, and availability. It also examines the techniques used to label and to weather reference and test MNPs prior to, during, or after production. Based on the research question investigated, the key MNPs properties to characterize are identified. This study aims to provide a baseline for the standardization of production and characterization of reference MNPs, which will ultimately facilitate the assessment of the risk associated with MNPs. |
1 Introduction
As an essential material in modern life, plastics have a wide range of applications, particularly polyethylene (PE, accounting for 27% of the global plastics production), polypropylene (PP, 19%), polyvinyl chloride (PVC, 13%), polyethylene terephthalate (PET, 6%), polyurethane (PU, 6%), and polystyrene (PS, 5%).1 Polyolefins (e.g. PE and PP) are typically used for food packaging, extrusion, or molding2 while PVC finds use in furniture, piping and in buildings.2 In contrast, PU has a wide range of applications such as building insulation, car seats and footwear. PET is employed in the production of plastic bottles and clothing. Native PS is used in packaging, while expanded PS foams are common insulation materials. With increasingly stringent environmental concerns and regulations there is also a push to use more naturally sourced and/or biodegradable plastics such as polylactic acid (PLA).In some specific applications plastics need to be manufactured in the micro- and nanometer size range, including microfibers for the textile industry, millimetric commodity pellets for manufacturing, micro, and nanoparticles for additive manufacturing, biomedical applications, paints, and personal care products.3–6 These small-sized plastics, referred to as primary microplastic (MP, >1 μm and <5 mm) and nanoplastic (NP, <1 μm) particles (referred to hereafter as MNPs)7,8 can enter different environmental matrices through various pathways.9–12 However, plastics usually enter the environment as much larger macroscopic objects either discarded at the end of their use phase or as debris from waste mismanagement. Due to their generally poor biodegradability, these macroscopic plastics persist in the environment and are embrittled and fragmented into secondary MNPs by weathering (e.g. by mechanical wear or photodegradation).13,14 It is these secondary MNPs that account for a majority of MNP pollution, as primary MNPs are becoming increasingly regulated.15,16
Due to their persistence and mobility, MNPs have been detected in almost every environmental compartment, including remote areas such as deep-sea trenches,17 mountains,18 and the Arctic.19 Further, recent reports show the presence of MNPs in plants20 and animals,21 as well as human blood,22 lungs23 and placenta.24 However, the accuracy of these studies is sometimes questioned. Indeed, one of the biggest reasons for the lack of clarity regarding the environmental fate and impact of MNPs stems from the fact that the identification and quantification of MNPs in different environments is extremely arduous. Due to the extremely low concentrations of MNPs in the environment, the first step in identification and characterization requires them to be concentrated and/or separated from the environmental or biological matrix following protocols that often comprise several digestion and filtration steps, which can lead to particle losses and contamination.25,26 The inherent challenges associated with these processes, combined with the lack of procedural standardization ranging from sampling to identification, make comparing results from different studies extremely challenging.26,27 Consequently, it is unclear whether the high variability in MNP concentrations reported in a given environmental compartment (e.g. water bodies, soil), in potential sources for human-exposure (i.e. food, beverages, personal care products, etc.), or the human body itself, reflects the inconsistency amongst analytical methods or actual MNP concentration variations.26 These considerable analytical difficulties result in a lack of quantitative information on MNP exposure and thus the risks posed by MNPs, although there is a widespread consensus that their concentration in the environment is increasing.15
These concerns have been responsible for a rapid increase in the number of studies evaluating the fate and effects of MNPs, including their toxicity, a topic which is intensely debated. On the one hand, the physical properties of the MNPs may be a key factor of their toxicity, for example in the flock worker's lung disease, similar to other hazardous non-plastic micro- or nanoparticles such as asbestos or silica dust.28–30 On the other hand, the chemical components within the plastics or adsorbed onto their surface, such as chemical additives or metals, also have the potential to significantly contribute to the hazards of MNPs.31,32 Indeed, it is unclear whether physical or chemical characteristics, or both dictate the toxicity of the MNPs because often some or most of them are neither controlled nor reliably reported.15,33 This example of toxicity is illustrative of the current challenges in MNP research. Moreover, variability is also observed in the results of ecotoxicity studies, which may be indicative of real differences, but also reflect inconsistent quality control or method validation.
One of the most significant difficulties in accurately measuring MNP exposure, fate and hazard is the limited accessibility to reference and test MNPs, because well-defined and varied MNPs provide an opportunity to ensure data quality and conduct controlled studies to identify the role that different physicochemical properties (e.g. size, shape, composition, surface properties) play in regulating key behaviors relevant to their environmental fate and effects. According to the terminology of the National Institute of Standards and Technology (NIST) and ISO Guide 30, the majority of MNP research is undertaken using research grade test materials, because MNP reference materials (sufficiently homogeneous and stable with respect to one or more characterised properties) are not readily available. Ideally, these MNPs should represent the main polymer types, particle sizes, shapes, surface properties and polymer compositions encountered in the environment. To effectively deploy reference and test MNPs in scientific studies, it is also important that a well-defined dose of these materials is homogeneously distributed into relevant environmental matrices, where they can serve either as a quality control (to exactly define recovery rates, detection limits, and analytical quality of a method)26 or as a tracer to study MNPs transport, fate, and effects. A survey of the literature reveals that a majority of studies employ commercially available, spherical, monodisperse, pristine particles, typically made of PS or PE, which may behave differently than fragmented, and aged particles found in the environment.34,35 Moreover, these materials are often costly and their manufacturers sometimes provide limited information regarding the homogeneity, stability, and presence of surfactants or additives in the plastics. These types of existing limitations have driven an increasing number of research groups to develop and use their own test and reference MNPs.
The importance of developing reference and test MNPs to improve the research quality in the field was recognized by a workshop of the American Chemistry Council in 2022. The stakeholders identified and summarized the needs and knowledge gaps for standardizing MNPs production and characterization methods,36 acknowledging that suitable production methods must be reproducible and with high yields. The two main methods of obtaining test and reference MNPs are chemical bottom-up and physical top-down approaches, each of which have innate strengths and weaknesses with respect to the range and properties of MNPs they can produce. To date, the majority of in-house produced MNPs are fragments, predominantly obtained by milling, a poorly defined process with a generally low yield for MPs of smaller sizes and NPs.37 Bottom-up methods generally offer better control over MNP properties and labels can be incorporated into MNPs during production to facilitate their detection or quantification, particularly in more complex matrices. In some MNP production methods uncontrolled aging can accompany the MNP manufacturing processes.
Despite the growing in-house production of reference and test MNPs, the capabilities, advantages, and limitations of the diverse existing methods have received limited attention.37,38 Besides, there is also a lack of appropriate characterization of the produced MNPs, tailored to the specific needs of different research studies. This deficiency is in part because the range of characteristics that fully describe a MNP is large, which poses a challenge given the limited range of analytical methods available to most researchers.
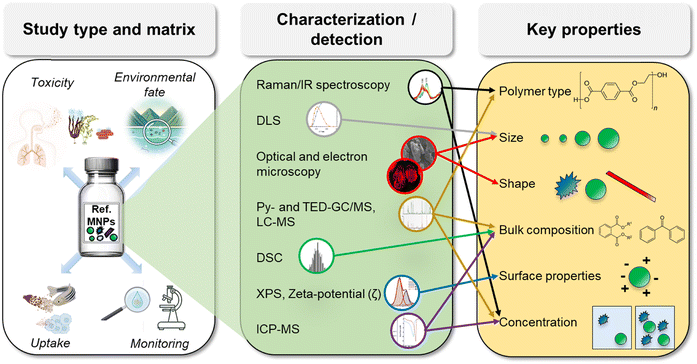
This review article assesses the current state of efforts directed towards the production, modification, and applications of reference MNPs and is a result of a 2023 workshop organized as part of The International Network For Researching, Advancing, and Assessing Materials for Environmental Sustainability (INFRAMES) program.§ Specifically, this paper aims to review: i) the main options to obtain test and reference MNPs, focusing on top-down and bottom-up production methods, ii) the possibility of mimicking the characteristics of the environmental MNPs by weathering, iii) the techniques for MNPs labeling to improve their detection, iv) the use of test and reference MNPs in the scientific literature, and v) the key properties of reference and test MNPs that should be characterized and how these depend on the specific research question. Although some discussion of MNP dispersion and the analytical techniques for MNP characterization and detection is included, these important topics are not the focus of this review. For more details on MNPs identification and quantification methods, the reader is directed towards several notable recent publications and review articles.39–42
2 Methods to obtain MNPs
Fig. 1 is a schematic of the principal routes researchers have used to obtain reference and test MNPs. Notably, reference and test MNPs can be obtained by: 1) direct purchase from a commercial supplier (commercial MNPs), including a few MNPs certified by metrology institutes; 2) collection and extraction from the environment; and 3) in-house production through top-down or bottom-up approaches in the laboratory. Commercial MNPs are the easiest to obtain, although this is the most expensive approach, and the MNPs are limited to specific polymer types, sizes, surface chemistries and shapes (ESI† 1). The most direct and representative approach, although challenging, is to extract MNPs from the environment. In controlled laboratory conditions, MNPs can be generated through size-reduction of macroscopic materials such as pellets, sheets, or environmental debris by physical means, typically mechanical (top-down methodologies, see section 2.3), or through chemical reactions and solvent separation processes (bottom-up, see section 2.4). Before, during or after production, lab-made MNPs can also be labeled to improve their traceability and quantification and/or weathered to improve their environmental relevance. In the following sections, we review the main methods that have been used to produce and modify reference and test MNPs. It should be noted that in this review the term “yield” is used to denote the mass of MNPs produced per unit mass of the initial material, whereas “production rate” is the mass of MNPs produced per unit time. | ||
| Fig. 1 Schematic overview of the main routes to obtain reference micro- and nanoplastic materials (MNPs). | ||
2.1 Commercial MNPs
In the last decade a number of commercial MNPs have become available and they have found widespread use in environmental studies.35,36,43,44 Despite their relative popularity, the choices of MNPs are often limited to monodisperse and spherical particles, obtained through reproducible, high-yield chemical methods (see section 2.4), though a few manufacturers offer fragmented variants. ESI† 1 contains a current list of commercially available MNPs, including information on the manufacturer, quantity, size, shape, polymer type, label if there is one, type of dispersion (suspension or powder), and price. This table is accessible in an online repository and intended to be continuously updated with new manufacturers and reference materials (see Data availability) The currently available MNP sizes range from tens of nanometers up to hundreds of micrometers, typically with low polydispersity values (variation coefficients less than 1%). The majority of commercial MNPs are restricted to a few polymer types, most notably PS and PE, which collectively represent over approximately one third of total global plastic production.1 In contrast, PP, PVC, PU, and PET which also constitute an important fraction of the total plastic production, are far harder to obtain as commercial MNPs. The range of commercially available MNPs available for use as test materials could be extended if primary MNPs were made accessible and their characteristics were known and reproducible. To aid in detection, some MNPs can be purchased with an embedded fluorescent, phosphorescent label, or enriched with a stable isotope (i.e.13C or 2H).Unfortunately, most MNPs cannot be considered reference materials according to NIST or ISO terminology. However, some new reference MNPs are currently under development as part of different projects and initiatives, so it is expected that in the near future the number of commercially available reference MNPs will grow. A very small number of commercial MNPs can be purchased from metrology institutes with certified properties; for example, standard reference materials (SRMs) can be obtained from the National Institute of Standards and Technology (NIST) consisting of spherical PS NPs with certified size (SRM 1964 – 60 nm ± 0.63, SRM 1963a – 101.8 ± 1.1 nm, SRM 1691 – 269 ± 4 nm, SRM 1690 – 895 ± 5 nm, and SRM 1961 – 29.64 ± 0.06 μm). Several companies offer NIST-traceable size standards of PS MNPs in the range of sizes from 20 nm to 160 μm (for example, Applied Physics). Interestingly, the German Federal Institute for Materials Research and Testing (BAM) offers micrometric cryogenically milled PS and PET fragments, and even cryogenically milled artificially UV-aged PE fragments. These particles are accompanied by a certificate of analysis of their morphology, size distribution, crystallinity, and surface chemistries, but are typically expensive, with prices reaching several hundred dollars per gram even for the cheapest ones (ESI† 1).
Certain characteristics of commercially available MNPs, such as the presence and concentration of organic or inorganic additives, surface properties, and stability are typically unknown or not reported, which complicates efforts to elucidate how these factors influence their behavior.42 Small-sized commercial MNPs are often sold as suspensions wherein the particles' stability has been enhanced by functionalization (e.g. carboxylated or aminated) or the addition of surfactants. The use of surfactants is strongly discouraged for test and reference MNPs because their presence could lead to surface interactions that differ from environmental MNPs. Indeed, Wieland et al.45 recently determined that nominally identical PS MPs acquired from different manufacturers differ significantly in their physicochemical properties, particularly zeta potential. This difference was likely due to the presence of various surfactants, initiators, and catalysts used during the synthesis, affecting particle–cell interactions. This study highlights the importance of characterizing MNPs “in-house” regardless of their nominal or reported physicochemical properties such that their behaviors can be meaningfully compared to other studies. One of the biggest differences between commercial MNPs and those encountered in the environment is that MNPs found in nature are invariably composed of polydisperse and irregularly shaped, non-spherical particles.34 As a result, there is no guarantee that the behavior of commercial MNPs is an accurate reflection or predictor of real-world MNP environmental fate and effect.
2.2 MNPs extracted from the environment
MNPs can be extracted from environmental samples and repurposed as reference and test MNPs in subsequent experiments.38,46,47 For instance, Waldschläger et al.47 collected floating particles from a river using a 5 mm mesh net and selected those that visually appeared plastic for further analysis with FTIR and Raman spectroscopy to confirm polymer types. To the best of our knowledge, this is the only study that collected actual MNPs (down to 580 μm) for subsequent use as test materials without subsequent size-reduction (e.g. milling of larger sized fragments).In studies aiming at reusing environmental MNPs as reference MNPs, extraction steps for purification and concentration will almost certainly be required because of the heterogeneous matrix and the likelihood that MNPs only constitute a very small fraction of the particles present. For example, MNPs may be intermixed with sand or sediment particles and/or be coated with an eco-corona. These coatings will likely need to be removed and the MNPs separated from the rest of the environmental constituents. Based on existing protocols, coatings are removed by oxidation with hydrogen peroxide, or alkaline, acidic, or enzymatic digestion, while mineral particles such as sand and sediment can be removed by sieving, density-based flotation, oil-based extraction,48–52 and electrostatic53 or magnetic54 particle extractions.40,48,55 To optimize purification and collection of environmental MNPs, these steps can be combined in different sequences depending on the complexity of the matrix. Regardless of the extraction chosen, it is crucial to preserve the integrity of the particles during the process and prevent unintentional aging or damage due to harsh chemical treatments. One possible solution to address this issue would be to use commercial MNPs as an internal control, subjecting them to the equivalent extraction process, and then characterizing the product to ensure that no alterations occurred during processing.51
In principle, extracted MNPs offer significant advantages as reference materials, because the particles are realistic and represent the complexity and diversity of particulate plastic pollution in the environment in terms of shapes, sizes, polymer types, and properties. However, given the complexity of these real-world samples, MNPs first require identification with spectroscopic methods. Unfortunately, the trace concentrations of MNPs in the environment necessitate the processing of large sample masses to obtain sufficient material. Moreover, given the complexity of these real-world samples, MNPs must first be identified and characterized with time-intensive spectroscopic methods. As a result, extracting and characterizing MNPs from environmental samples can take days to weeks,48 in contrast to in-house production methods, which usually require just a few hours. Moreover, the extraction processes have the real possibility of producing unintended artificial weathering of the MNPs. For these reasons, the extraction of MNPs as reference and test materials from realistic environmental samples is extremely challenging, severely limiting the overall utility of this methodology. This is reflected in the lack of popularity for this approach.56
2.3 Top-down production
Top-down approaches encompass mechanical and thermal methods that physically reduce the size of plastics to generate MNPs. The principal top-down techniques used by researchers, shown in Fig. 2, are (cryo-)milling, sonication, laser ablation, sanding, and (cryo-)microtomy. These will be described in more detail in the following sections. Table 1 summarizes the main reports using top-down methods to produce MNPs as well as the key parameters that define the method (procedure, origin of the plastic, polymer type, size, morphology, pre- or post-production labeling). | ||
| Fig. 2 Schematic of top-down approaches used to produce MNPs, including (A) milling and grinding, (B) sonication, (C) laser ablation, (D) sanding, (E) extrusion and microtomy. | ||
| Production method | Labeling | Starting material | MNPs morphology | MNP size | Characterization techniques | Advantages | Limitations | Ref. |
|---|---|---|---|---|---|---|---|---|
| Blade grinder and planetary ball mill | PS (Goodfellow) | Fragments | 0.07 μm (PE) | ζ, DLS, AF4, ATR-FTIR | • Method reproducibility | • High affinity for common polymeric membrane filters | 57 | |
| PE (Total) | 0.3 μm (PS) | |||||||
| Cryomilling and cascade sieving | PBAT, Grade EF04P (Mater-Bi) | Fragments | 5.9–500.7 μm (PBAT) | DLS, TGA, FTIR, DSC, GPC | • Simple and effective | • Small yield (5%) on the later fraction (45 μm) | 58 | |
| LDPE (Dow Chemical) | 591.0–43.2 μm (LDPE) | • Extended to other plastic families | ||||||
| • Employed to investigate kinetics and time course of size reduction, salinity | ||||||||
| Blade milling in liquid nitrogen | PET (Indorama, Polymers Europe) | Fragments | ∼60 μm | FE-SEM, Raman | • Fast | • Loss of crystallinity during milling | 59 | |
| • High yield | ||||||||
| • Simple | ||||||||
| • Accessible | ||||||||
| Blade milling in liquid nitrogen | PLA (ErcrosBio LL 650) | Fragments | 240 ± 65 μm | FE-SEM, Raman | • Fast | 60 | ||
| • High yield | ||||||||
| • Realistic MPs | ||||||||
| Cryomilling | PP, PMMA (LG Chem.) | Fragments | 50–300 μm | FE-SEM, Raman, DSC | • Fast | • Bulk material may be affected during synthesis | 61 | |
| PET (LOTTE Chemical) | • High yield | |||||||
| • Realistic MPs | ||||||||
| Cryogenic grinding and wet-sieving | PVC (Flexfilm) | Fragments | 75–2000 μm | LD | • Fast | • Broad size distribution | 62 | |
| • Extended to other plastic families | • Large particles only | |||||||
| Cryogrinding with ball mill and sieving | PE | Fragments | Polydisperse | Coulter counter, LD, SEM | • Fast | • Reproducibility is limited | 63 | |
| PP (ocean) | • High yield | |||||||
| • Realistic MPs | ||||||||
| Centrifugal milling | PP (Lyondell Basell) | Fragments | 1–180 μm | SLS, SEM-EDX, TGA, XRF | • Many monodisperse size fractions | • Possible talc contamination | 64 | |
| Cryomilling with a ball mill and sieving | PVC (Sigma-Aldrich Merck) | • Stable particles | • Only for plastics with density >1 | |||||
| Cryomilling, sieving, oxidation with ozone in water | PS | Fragments | 1–100 μm | SEM, Coulter counter | • Extended to other plastic families | • Broad size range | 65 | |
| • Specialized equipment | ||||||||
| • PS oxidation | ||||||||
| • Generation of dissolved organic compounds | ||||||||
| Cryomilling with a ball mill | PS (culture test tubes, Fisherbrand) | Fragments | 250 nm | ATR-FTIR, SEM, Coulter counter, NTA | • No aging during cryomilling | • Low yield (<0.1%) | 66 | |
| • Fast | ||||||||
| Cryomilling | NR, NB staining | PA (Lanxess) | Fragments | 90–125 μm | Optical microscopy, DLS | • Fast | • Size and shape heterogeneity | 67 |
| PET (TPL) | • High yield | |||||||
| PLA (Nature Works) | • Realistic MPs | |||||||
| PS, LDPE, PVC (Ineos) | ||||||||
| PMMA (Röhm) | ||||||||
| PP (Borealis) | ||||||||
| Milling and sieving | PET | Fragments (flakes) | 5–60 μm | ATR-FTIR, FE-SEM | • Fast | 68 | ||
| 61–499 μm | • High yield | |||||||
| 500–3000 μm | ||||||||
| Mechanical abrasion via stirring and ultrasonication | PP, PE (environmental debris) | Fragments | 120–180 nm | DLS, TEM, XPS, BET, Py-GC/MS, ATR-FTIR | • Simple | 69 | ||
| 600–800 nm | • High yields (80–90%) | |||||||
| • High concentration (∼400 mg·L−1) | ||||||||
| Crushing, grinding, milling, and aging with UV and ozone sonication | PS (single-use forks) | Fragments | 90–100 μm | SEM-EDX, ATR-FTIR, ζ, DSC, XRD, BET | • Fast and simple aging protocol | • Size distribution limited to 1–5 μm or ∼100 μm | 70 | |
| 1–5 μm (after aging) | •Monodisperse | • Only PS | ||||||
| • Artificially and naturally aged particles similarity | ||||||||
| Alkaline ultrasonication | PLA | Fragments | 30.2 ± 12.1 μm (PS) | Raman, ATR-FTIR, μ-FTIR, SEM-EDX | • Size and shape reproducibility for PLA and PET | • Slight color change | 71 | |
| PS | Fibers | 32.0 ± 6.5 μm (PLA) | • Efficient to produce aged particles | • Potential alteration with KOH | ||||
| PET | Films | 26.6 ± 5.2 μm (PET) | • High yield and production rate | • Poor reproducibility for PS, PE, PP, PVC and PA | ||||
| PVC | • Poor interlab reproducibility | |||||||
| PE | • PLA and PS are oxidized | |||||||
| PP | ||||||||
| PA (daily life products) | ||||||||
| Fragments ground with household blender | PE | Fragments | ATR-FTIR | • Fast | • Large particles (>100 μm) | 72 | ||
| Fibers cut with a scalpel and encapsulated in methylcellulose or gelatine | PP | Fibers | 100–500 μm | • Simple | • ATR-spectra suggest modification of the plastics | |||
| PS | • Affordable | |||||||
| PES (daily life products) | ||||||||
| Paraffin wax embedding and microtomy | NR staining | PET | Fibers | 14.7 ± 4.8 μm | Optical microscopy | • Fast | • Microtome availability | 73 |
| PA6,6 (Goodfellow) | 28.4 ± 3.6 μm | • Target size is precise, repeatable and modulable | ||||||
| 41.3 ± 3.5 μm | ||||||||
| 11.8 ± 5.1 μm | ||||||||
| 30.2 ± | ||||||||
| 2.9 μm | ||||||||
| 108.3 ± 24.3 μm | ||||||||
| Extrusion and microtomy or cryomilling | HDPE, PP, PS (LG Chem) | Cylinders | 74–133 μm (cylinders) | Optical microscopy, FE-SEM, FTIR, DLS, Py-GC/MS | • Simple method | • High polydispersity | 74 | |
| PET (Lotte Chemical) | Fragments | ∼10–100 μm (fragments) | • Suitable for any plastics | • Filtration | ||||
| PA6 (Hyosung TNC Co.) | • Cylinders not environmentally relevant | |||||||
| Cryotomy | NR staining | Nylon (PA) | Fibers | 40, 70, 100 μm (length) | Optical microscopy, SEM | • Monodisperse | • Limited to fibers <100 μm | 75 |
| PET | Aspect ratio >1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
• Targeted size | • Requires −80 °C | |||||
| PP (Goodfellow) | • Cheap and quick (∼60k fibers per h) | • Freezing might modify the properties | ||||||
| Microtomy | PA6 | Fibres | 200 μm (length) | ATR-FTIR | • Suitable for fibers from different origins | • Possible variability of fiber length should have been considered in the detection probability | 76 | |
| PA6.6 | ||||||||
| PET | ||||||||
| PP (Aquafil S.) | ||||||||
| Laser ablation and filtration | PC | Spheres with irregular surface | 50 nm | DLS, TEM, XPS | • Monodisperse nanoplastics | • Formation of carboxyl and hydroxyl group during ablation | 77 | |
| PET (Goodfellow) | • High production rate | • Change of surface charge | ||||||
| • Suitable for any plastics | ||||||||
| Laser ablation and rotavap | PET (Goodfellow) | Fragments | 26.7 ± 14.2 nm | TEM, DLS, AF4, ζ, XPS, spectrophotometer for CCC and CSC evaluation | • Extended to other plastic families | • Oxidation | 78 | |
| Spheres | • Controllable polydispersity and size | • Laser needed | ||||||
| • Low yield | ||||||||
| • Small size |
A wide range of plastics can be milled using readily available household instruments. For example, PE, PS, PES, PVC, PET, and PP fragments of hundreds of micrometers in size were generated using a kitchen grinder.72,79 An immersion blender was used for between 10–120 min to grind PP, PS, and PE fragments into similar sizes of a few hundred micrometers.80,81 Similarly, Ekvall et al.82 used an immersion blender for 5 min, followed by a <0.45 μm filtration step, and observed PS nanoplastics in the 125–437 nm range. Although the yield was not reported, it is reasonable to assume that only a very small quantity of NPs was produced. Relevant experimental parameters such as the initial material size, the blade rotation per minute, or the blending time are generally not provided, making it challenging to reproduce and optimize these methods. While not reported in the literature, it is worth mentioning that contamination of the MNP surface due to the various plastic or metal parts of the kitchenware may also occur, although this potential issue has not been explicitly addressed.
Due to the viscoelastic properties of plastics, grinding results in the deformation of the material and inhibits complete breakdown.59 To overcome this limitation, milling is often carried out under cryogenic conditions using liquid nitrogen to impart brittleness to the plastic. This process, referred to as cryogenic milling or grinding, facilitates size reduction by preventing melting caused by the frictional heat released during milling.58,64,66 Cryomilling also reduces the size polydispersity of the resulting material in comparison to room-temperature conditions, and the size distribution can be further modulated by varying the parameters used to cryomill the MNPs.64,66 Thus, Eitzen et al. (2019) evaluated the effect of liquid nitrogen cooling time prior to milling on the size distribution of PS granules.65 Milling without pre-cooling resulted in 90% of the milled PS mass being composed of particles larger than 100 μm. When the pre-cooling time was increased to 12 min, only 45% of the particle mass was composed of particles larger than 100 μm, the rest being smaller than 100 μm. The same study also evaluated the impact of the number of milling cycles on the mass distribution of PS MNPs.65 After one 10 min cycle at 30 Hz, more than 95% of the PS MNP mass was in excess of 100 μm. Increasing the number of milling cycles to more than three resulted in a mass fraction dominated by particles of sizes between 50 and 100 μm.65 Overall, these results highlight the potential to control size distributions of milled MNPs, although the impact of other milling parameters (e.g. rotation speed, time, or quantity of material under milling, etc.) on particle size distribution has yet to be investigated systematically. These efforts would also benefit from more thorough reporting of mass, volume, and size distribution of resulting MNPs.
In addition to size distribution, it is important to characterize the physicochemical properties of ground or cryomilled MNPs. Although Smith et al. (2024) did not observe any measurable changes in the surface chemistry or chemical bonds in cryomilled PS, PMMA, PE and PVC MNPs compared to the nascent polymers, as determined by XPS and ATR-FTIR, some changes in the physical properties of MNPs after milling have been reported.83 For example, Jiménez-Arroyo et al. (2023) observed a change in intensity of the 397 vs. 410 cm−1 Raman bands for cryomilled micrometric PLA, indicative of a higher amorphicity and a broader crystallinity distribution compared to the as-received PLA millimetric pellets.60 Similarly, Tamargo et al. (2022)59 noted a slight loss in crystallinity for cryomilled PET compared to the initial material due to particle cracking, embrittlement, and surface fracturing associated with compression during cryogrinding. Cuthbertson et al. (2024)84 also reported changes of both crystallinity and molecular weight due to cryomilling. Unfortunately, the impact of milling/cryomilling on MNP properties is rarely reported. More systematic data and understanding is certainly needed in this area, particularly given the widespread use of this technique to create MNPs.
The popularity of grinding, blending, milling and cryomilling as means to create MNPs can be attributed to the apparatus being fast, widely available, inexpensive, and capable of generating MNPs for a variety of polymer types. However, the substantial polydispersity of the resulting material and low particle yield58,66 are limiting factors, particularly for nanoplastics generation.
Blancho et al. (2021) agitated and sonicated beach and sea plastic debris made of PE and PP, which generated nanosized fragments (∼150 to 500 nm) reaching a concentration of 400 mg L−1 in the resultant solution.69 The yield, which ranged from 80 to 90% of the mass of particles <1 mm, was remarkably high for nanoplastic production using a top-down technique, and was likely due to the use of weathered and potentially embrittled plastics. Similarly, Enfrin et al. (2020) extracted PE nanoplastics from facial scrub products (∼400 nm) and subjected them to ultrasonication, generating nanosized particles (∼50 nm).86 The amount of NPs produced (1013 to 1014 particles per g of initial material) correlated with the energy input of the sonicator, demonstrating the potential to optimize the generation of NPs.
Given the common availability of ultrasonic baths in many laboratories, this is an ideal and somewhat underutilized method for producing reference and test MNPs, albeit ones that are possibly aged.
Similar to ultrasonication, laser ablation may well modify the surface properties of the plastic. For example, an increase in the O/C ratio of PET and PC MNPs, and the formation of carboxyl and hydroxyl groups have been reported, likely a consequence of photooxidation.77,78,88,92 Given the nature of the production method, which in some sense amounts to a form of accelerated aging, laser ablation can be considered a viable means to produce spherical, weathered MNPs. Overall, laser ablation can produce monodisperse MNPs in the nanosized range in a relatively short time, but the specialized nature of the equipment as compared to milling or grinding for example means that this method has yet to find widescale implementation.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.7 While fibers can be more easily distinguished from other particles in the environment due to their characteristic shape, micro- and nanofibers are not to the best of our knowledge commercially available. Therefore, there is a need to develop laboratory-based methods to create this class of MNPs. The production of micro- and nanofibers usually is a two-step process where first a long polymer filament is generated through extrusion, such as melt-spinning,97,98 which is then cut at regular intervals.
3.7 While fibers can be more easily distinguished from other particles in the environment due to their characteristic shape, micro- and nanofibers are not to the best of our knowledge commercially available. Therefore, there is a need to develop laboratory-based methods to create this class of MNPs. The production of micro- and nanofibers usually is a two-step process where first a long polymer filament is generated through extrusion, such as melt-spinning,97,98 which is then cut at regular intervals.
The general approach to produce long polymer filaments is to melt plastic pellets or powders before extruding them. This high throughput technique has been applied to PHBV, PLA, and PET and produced kilogram quantities of filaments per hour with a diameter of ∼30 μm.97,98 Electrospinning is another extrusion process, where a liquid polymer (either dissolved or melted) passes through a spinneret and is elongated by an electric field, forming a thin filament. It is important to note that the filaments are typically collected as complex non-woven networks and rarely as a single filament.99 This process can be performed with a simple apparatus, enabling the fabrication of fibers typically as small as a few hundred nanometers in diameter for multiple polymer types – including PS, PVC, PLA, PP, PE, PU, nylon 6,6, PMMA, and PET.99 Furthermore, the characteristics of the filament are easily controllable.99 For instance, the diameter of the fiber can be tuned by adjusting specific parameters such as the voltage applied or the flow rate of the polymer solution. The throughput of about 1 g h−1 is reasonably high, although the resulting filament must still be cut to obtain fibers. While electrospinning demonstrates significant potential, particularly for nanofiber production, the generation of reference fibers with such a method has not yet been reported.
Common approaches to cutting the resulting filament into micro- and nanofibers involve ball-milling or manual cutting with a kitchen knife.98,100,101 However, the fibers produced are typically longer than 100 μm, polydisperse, and exhibit a high degree of sample-to-sample variability. Alternatively, a microtome, a cutting tool that prepares thin sample sections, has been used to more precisely reduce the length of the microfibers.75 To implement this tool, the thin plastic filaments are aligned before being placed in the microtome and sliced at regular intervals. At room temperature, alignment of the filaments can be accomplished by placing them in a wax such as paraffin that can subsequently be dissolved in an organic solvent to leave behind an aligned bundle of fibers.73 Another option is cryomicrotomy, which involves aligning the plastic filaments by using a freezing agent at −80 °C, before the filaments are cut with the microtome.75 Cryomicrotomy and microtomy can successfully cut most common plastics found as microfibers (PET, PP, and nylon 6,6) to targeted lengths, from 10 to 100 μm.73,75 The cutting process allows for the production of fibers at rates ranging from 105 to 106 fibers per h. Although microtomy has only been applied to cut microfibers down to lengths as small as 10 μm,73,75 there is no reason a priori why this approach could not be extended to produce nanofibers, provided that plastic nanofilaments are available. As a final note, we have classified the generation of fibers as a top-down method because the main challenge is to cut the resulting filament at very small intervals rather than the extrusion process, which is a well-established industrial bottom-up process.
The yields and production rates of top-down methods, though not always reported, are reasonable for particles larger than ∼1 μm, but unfortunately low for nanosized particles. All top-down techniques can be applied to both pristine and aged plastics. There is a suggestion that weathered plastics are more amenable to fragmentation by strong mechanical (such as milling and sanding) or chemical (sonication) stress as compared to their pristine counterparts,70,102 potentially opening up a route to higher MNP yields.
Alteration of MNPs as a consequence of using top-down methods, due to contamination or oxidation of the plastic surface, has sometimes been reported, highlighting the need to systematically investigate the unintentional changes that can occur during fragmentation. Indeed, some top-down methods do produce weathered MNPs, and metal contamination, including Zr and Y with milling,64 Al with sanding93 or Pb, Ti, Fe, and Al with ultrasonication.70 This contamination is due to material transfer from the apparatus, so the use of metal-free parts must be prioritized, when possible, because this type of surface contamination may be critical, for example in artificially skewing MNP toxicity measurements. In contrast, induced surface changes (excluding metal transfer) can help to mimic real MNPs characteristics, since the majority of MNPs in the environment are secondary MNPs formed by the physical breakdown of plastic debris. Therefore, top down approaches seem to be the most relevant to produce reference and test MNPs for environmental and human impact studies. However, it must be noticed that nanoplastics generated by mechanical routes have been reported not only to be oxidised, but also to have a low surface charge, facilitating their stable dispersion in water.103 One of the biggest challenges in creating true-to-life MNPs is understanding how to mimic as closely as possible the environmental processes. In this line, a very recent publication explores the effects, advantages and limitations of three distinct cryogenic grinding techniques (ultracentrifugal mill, immersion blender, mixer mill).104
In conclusion, further investigation of ultrasonication and sanding methods are warranted due to their efficient production of monodisperse low micron-sized and nanosized particles. Given that most studies utilizing grinding and milling often lack a clear description of the methodology employed, we encourage future studies to publish a detailed procedure and to attempt fine-tuning milling parameters in order to obtain specific size distributions and reduce polydispersity. This additional information could enable the modeling of the size distributions of the resulting material as a function of the instrument parameters (i.e. precooling time, milling frequency, etc.). More easily accessible top-down methods that can create reference and test nanofibers would be welcome as the study of these high aspect ratio MNPs is severely lacking.
2.4 Bottom-up production
Bottom-up methods involve the controlled production of MNPs from 1) smaller building blocks, typically by polymerization of monomers, or 2) by solvent removal or precipitation of MNPs from solutions of dissolved macroscopic plastics (Fig. 3). Table 2 summarizes the main reports using bottom-up methods to produce MNPs along with the key information related to their production and properties (technique used, origin of the plastic, polymer type, size, morphology, labeling). These chemical processing approaches offer significantly more control over the size and composition of the resulting MNPs, typically in the nano- or low micron-sized range as compared to top-down methods (Table 2). The high yields and reproducibility associated with these methods are well suited to scale-up MNP production. Moreover, there is no uncontrolled MNP aging associated with bottom-up methods, and in some instances, controlled surface functionalization can be performed during or post-production, for example, with carboxyl groups introduced by the addition of acrylic acid during the polymerization. The presence of these surface functional groups can mimic MNP aging105 or provide anchoring points for subsequent metal labeling.106 Labels can also be incorporated into the bulk of MNPs during bottom-up production (e.g. metal or fluorescent cores), as described in the next section. Bottom-up methodologies also offer the possibility of incorporating specific additives or producing additive-free MNPs.107 One disadvantage of bottom-up methods is that the shape of the MNPs is typically spherical (Table 2), in contrast to the irregularly shaped MNPs encountered in the environment. Another important issue may arise from the possible incomplete removal of surfactants, solvents, precursors, or initiators employed in some bottom-up approaches. These species may lead to erroneous conclusions38,108,109 about MNP properties, so a detailed analysis of the liquid phase wherein the MNP is suspended at the end of the synthesis is strongly encouraged, but this is rarely reported. | ||
| Fig. 3 Schematic representation of the two main routes for the bottom-up production of micro and nanoplastics (MNPs). | ||
| Production method | Labeling | Starting material | MNP morphology | MNP size | Characterization techniques | Advantages | Limitations | Ref. |
|---|---|---|---|---|---|---|---|---|
| Emulsion polymerization and conjugation with functionalized gold-containing nanoparticles | Au | PS, (Polysciences, Warrington, PA) | Spherical | 15 ± 5 nm (Au) | SP-ICP-MS | • Detection and quantification of nanoplastics in aqueous media | • Approach not directly applied in more complex matrices presenting negatively charged species (organic dissolved matter and/or other particles) | 106 |
| 759 ± 23 nm, 990 ± 20 nm, 3030 ± 121 nm (PS) | SEM | • Carboxyl groups at MNPs surface mimic plastic aging | ||||||
| • Conjugation with functionalized positively charged AuNPs | ||||||||
| One-step polymerization and direct radiolabeling of a sulfonate end-capped nano-sized PS | 14C | PS | Spherical | 20.0–47.5 nm | Biomolecular imaging (autoradiography) | • NPs trackable in simulated environmental settings | • Low accessibility to autoradiographic equipment | 110 |
| ATR-FTIR | • Yield of production of 84 ± 28% | • Unrealistic scenario with synthesized nanoPS | ||||||
| • Necessity to study the behavior of synthetic nanoPS vs. PS in the environment | ||||||||
| • Limited characterization possible because of radioactive label | ||||||||
| Synthesis of a polyacrylonitrile core containing Pd followed by addition of a cross-linked PS shell | Pd entrapment | PS | Spherical (raspberry-like if synthesis conditions are modified) | 150–190 nm | SEM | • Techniques pre-developed for measuring inorganic nanoparticles can be used | • Metal tracer alone cannot provide size, shape, or polymer identity information | 111 |
| TEM | • Lack of leaching of metal tracer indicates suitability for ecotoxicity tests | |||||||
| STEM-EDX | ||||||||
| DLS | ||||||||
| ICP-MS | ||||||||
| Synthesis PS and P2VP copolymer intercalated with metal nanoparticles and capped with polymeric shell | Au, Pt, Pd entrapment | PS-P2VP | Spherical | 300–500 nm | DLS | • Techniques pre-developed for measuring inorganic nanoparticles can be used | • Metal tracer alone cannot provide size, shape, or polymer identity information | 112 |
| PMMA-P2VP | AFM | • Lack of leaching of metal tracer indicates suitability for ecotoxicity tests | ||||||
| HAADF-STEM | ||||||||
| SEM-EDX | ||||||||
| FFF | ||||||||
| ζ | ||||||||
| FTIR | ||||||||
| TGA | ||||||||
| Raman | ||||||||
| spICP-MS | ||||||||
| In situ growth of Au nanoparticles on NPs surface | Au nanoparticle adsorption | PS | Spherical | 50–1200 nm (PS) | TEM | 113 | ||
| PE | 0.2–9.54 μm (PE) | SEM-EDX | ||||||
| PVC | 0.21–1.54 μm (PVC) | XPS | ||||||
| PET | 0.12–53.63 μm (PET) | NTA | ||||||
| PP | 160–582.5 nm (PP) | spICP-MS | ||||||
| PMMA | 400 nm (PMMA) | |||||||
| Free-radical polymerization | PE | Spherical | 30–110 nm | DLS | • Works without surfactant | • Lower yield without surfactant | 114 | |
| TEM | • Lower polydispersity without surfactant than with | |||||||
| NMR | ||||||||
| Free-radical polymerization | PE | Spherical | 10–110 nm | DLS | • Fast process (4 h) and important production possible (grams) | • Surfactant (SDS) | 115 | |
| Cryo-TEM | • Yields up to 30% | |||||||
| SEC | ||||||||
| NMR | ||||||||
| DSC | ||||||||
| Nickel(II)-catalyzed emulsion polymerization | PE | Disc-shaped | 30–300 nm | DSC | • Monodisperse | • Rather low molecular weight (∼103 g mol−1) | 116 | |
| DLS | • Potential nickel contamination | |||||||
| TEM | ||||||||
| AFM | ||||||||
| Polymerization with Ziegler–Natta catalyst | PP | Spheres | <500 nm, 1 μm, 5 μm, 10 μm, 20 μm | SEM | • Monodisperse particles in the micrometer size range | • Potential metal contamination (Al, Ti) | 117 | |
| TEM | ||||||||
| AFM | ||||||||
| XRD | ||||||||
| FTIR | ||||||||
| DSC | ||||||||
| Dissolution and emulsification with a surfactant or biosurfactant | NR added to solution | PE (CTTM) | Fragments | 200–800 nm | TGA | • Simple, cheap and fast for NP | • Density of final product is higher than the initial ones | 118 |
| DSC | • Labelling possible | • High polydispersity | ||||||
| High yield with surfactant | Use of a surfactant | |||||||
| Dissolution and precipitation in water | Rhodamine B added to solution | PET (IZO HomeGoods) | Spheres | 95 ± 14 nm (no fluorescence) | DLS | • Stable particles | • Produced nano PET different than the initial one | 119 |
| 88 ± 14 nm (with fluorescence) | ζ | • Monodisperse | • Only valid for PET | |||||
| ATR-FTIR | • Simple method | |||||||
| 19F-NMR | ||||||||
| TEM | ||||||||
| SEM | ||||||||
| UV-vis | ||||||||
| Raman | ||||||||
| Py-GC/MS | ||||||||
| XPS | ||||||||
| Dissolution and reprecipitation with surfactants | LDPE | Spheres | 40 nm (LDPE) | FPA-FTIR | • Simple protocol using common reagents | • Could not recognize IR spectra of PET | 120 | |
| HDPE | 50 nm (PVC, PET) | NTA | • Monodisperse | • Surfactants might affect surface properties | ||||
| PVC | 70 nm (HDPE, PP) | SEM | ||||||
| PP | 200 nm (PS) | |||||||
| PS | ||||||||
| PET (Goodfellow) | ||||||||
| Dissolution in different solvents and nanoprecipitation with DMSO, water/DMSO or water/ethanol | LDPE pellets (Sumitomo Seika Chemicals) | Spheres | 426–463 nm (LDPE, HDPE, PP) | Optical microscopy | • >90% of NPs | • Crystallinity of reference particles lower than commercial polymers | 121 | |
| LDPE sheets (KOKUGO) | 180 nm (PVC) | GPC | • Properties generally similar to commercial polymers | |||||
| HDPE rods (Alfa Aesar and ESCO) | 226 nm (PS) | DSC | ||||||
| PP pellets (Sigma-Aldrich) | SEM | |||||||
| PVC powder (FUJIFILM Wako Pure Chemical Corporation) | ||||||||
| PS sheets (Goodfellow) | ||||||||
| Impinging jet mixing process for nanoprecipitation or nanoemulsion, followed by cryomilling | NR, fluorescein, rhodamine B added to solution | PET granules (Goodfellow) | Spheres | PET (31 nm) | AFM | • High yields | • Surfactant needed | 107 |
| PS granules (Sigma-Aldrich) | Fragments | PS (71 nm) | SEM | • Stable NPs | ||||
| PE flakes (Sigma-Aldrich) | PE (32 nm) | DLS | • Small-sized particles | |||||
| PP granules (Sigma-Aldrich) | PP (31 nm) | ζ | • Easy-to-add fluorescent tags and chemical additives | |||||
| DSC | • Simple setup-up | |||||||
| TGA | ||||||||
| Dissolution of ball-milled plastics and nanoprecipitation | PET (plastic bottles) | Spheres | 90 nm | DLS | • Monodisperse | • Surfactant | 77 | |
| TEM | • No surface oxidation during synthesis | • Ball-milling before dissolution | ||||||
| XPS | ||||||||
| Dissolution and nanoprecipitation | PET (plastic bottles) | Spheres | 69, 79, 80, 85, 108, 126 and 154 nm | DSC | • Controlled size-distribution | • Not environmentally relevant shapes | 122 | |
| PET films (Goodfellow) | DLS | • No surfactant | ||||||
| FE-SEM | ||||||||
| Dissolution and nanoemulsion | Au, Pt, octaethylporphyrin, quantum dots added to solution | PP (Sigma-Aldrich) | Spheres | 80–350 nm | SEM | • 90% yield | • Shapes not environmentally relevant | 123 |
| EDX | • High production rate | |||||||
| spICP-MS | • Can easily add different tags | |||||||
| DLS | • Reproducible | |||||||
| Raman | • Monodisperse | |||||||
| • Good colloidal stability | ||||||||
| • Signal of NPs is similar to bulk polymer | ||||||||
| Dissolution and nanoprecipitation with SDS | NR staining | PET (drink bottle) | Fragments | 224 nm | TEM | • Monodisperse | • Surfactant (SDS) | 124 |
| DLS | • Large production rates | |||||||
| ATR-FTIR | ||||||||
| Dissolution of PET in TFA, stirred and centrifuged. Precipitate resuspended in SDS and ultrasonicated | PET (bottles) | Spheres (PS) | 200, 500, 1000 nm (PS) | AFM | • High sensitivity and reliability in the trace nanoplastics detection | 125 | ||
| PET (synthesized) | Fragments (PET) | <200 nm (PET) | Raman | |||||
| PS (Sigma-Aldrich) | NTA | |||||||
| SEM | ||||||||
| Dissolution and precipitation | PA-6 (pellets) | Fragments | 0.102 μm (PA-6) | DSC | • High yields and production rate | 126 | ||
| LDPE (Lupolen) | 11–50 μm (LDPE) | Analytical centrifugation | • Irregular morphologies | |||||
| PET | 1–7 μm (PET) | FTIR | ||||||
| PU | 1.03 μm (PU) | SEM | ||||||
| He-pycnometry | ||||||||
| Electron paramagnetic resonance | ||||||||
| ζ | ||||||||
| Headspace-GC |
To some extent, the bottom-up synthesis of MNPs leverages existing knowledge on creating polymeric NPs for drug delivery in biomedical applications.3,4 At the same time, significant current efforts are being directed towards developing new innovative methods and refining existing techniques to achieve better control over MNP properties such as particle size, shape, or crystallinity. Fig. 3 shows the two main bottom-up strategies for creating reference MNPs that are summarized in the next sections: polymerization reactions and solvent removal/precipitation, both in emulsions or suspensions.
Polymerization has also been used as part of a 3D printing process to produce MP fibers of different sizes, shapes and aspect ratios. In this process, an acrylic resin is polymerized with spatial specificity into MP fibers using a two-photon lithography technology. The density of the resultant fibers is similar to that of many common MNPs (PP, PS, HDPE).129
3 MNP labeling
One of the biggest challenges in MNP studies lies in the difficulty associated with their identification and quantification in realistic, heterogeneous environmental matrices where the overwhelming majority of species are not MNPs (e.g. silica, clays, or natural organic matter). For this reason, several methods have been developed to prepare reference and test MNPs that are easier to identify and quantify. Specifically, these methods typically involve the addition of a label or tag to improve the ability to detect and differentiate test MNPs from other naturally occurring species without the need for large sample collection or complex extraction procedures. Furthermore, labeled reference materials can also be applied as internal standards, positive controls or controls for method validation. These labels, tags, or tracers (Fig. 4) are typically incorporated either after MNP production, by direct application of the label to the surface of existing MNPs, or during MNP production, by incorporation of the label onto the surface or into the bulk during the bottom-up preparation of the MNPs. An alternative would be to use a labelled plastic as the starting material, although this is very rare. Ultimately, the selection of which labelling approach to use will depend on the particular application and properties of the label (e.g., limit of detection associated with a particular label) and the accessibility of each labelling method to a particular researcher. In this section, current lab-based labeling techniques applied to MNPs will be discussed alongside their respective advantages and disadvantages.3.1 Fluorescent labeling
Fluorescent dye labeling is one of the easiest and most cost-efficient techniques to prepare labeled MNP materials, and thus is the most widely used. Fluorescent tags are added in one of two ways: by physical adsorption of a fluorescent dye onto the MNP surface (staining) or by addition of a fluorophore to the polymer matrix during bottom-up preparation of MNPs. When a MNP is fluorescently labelled the fluorescent signal enables the visualization of the MNP, typically by fluorescent microscopy, but also by flow cytometry134 and photoluminescence-based techniques. Although certain plastics do naturally fluoresce, the degree of fluorescence is rarely sufficient to enable visualization. The staining procedure can also be directly applied to environmental samples; however it is insufficient for the quantification of MNPs in environmental samples because visualization of fluorescent particles does not guarantee that these particles are plastic.135,136One commonly used and straightforward method for fluorescently-labelling MNPs is via surface staining with Nile red (NR). This hydrophobic dye enables the visual differentiation of MNPs from interfering hydrophilic particles such as sand in environmental samples. A recent review has summarized the dyes used for detection of MNPs.135 While NR is the common choice, Rose Bengal135 and pink textile dyes137 are less expensive alternatives. It should be noted that some polymers including PC, PU, PET, and PVC, have been shown to fluoresce only weakly after staining with NR, limiting its applicability to lower density (and generally more hydrophobic) plastics. A typical protocol involves adding the stain to MNPs on filter paper followed by washing and drying to remove excess dye.138 Careful consideration of the carrier solvent for the dye must be applied to ensure compatibility with the MNPs. Additionally, samples must be pre-treated to remove undesired natural organic matter that could bind to the dye and cause false MNP signals. The advantage of this method is that it provides a high throughput means to prepare easily identifiable MNPs of lower-density polymers such as PS, PP, and PE.139 In an alternative approach, Caponetti et al. (2021) have developed a detection system using a probe based on perylene-diimide to label different types of MPs, including PVC, PE, PET, PP, PS, PMMA, and PTFE. The probe exhibited high selectivity for PVC, with intense red emission after labeling, providing an environmentally friendly, sensitive, low-cost approach for MNP detection.140 Since staining is restricted to the MNP surface, the fluorophore concentration, and thus the fluorescence signal of each particle, is limited. Further, many fluorophores are not photostable, which compromises the labeled MNP storage and their application in photolysis studies designed to examine MNPs behavior in the presence of light. Moreover, MNP surface properties could be altered by adsorption of the dye, which would cause the measured properties to not reflect those of the unamended MNPs. An alternative to adsorption is to infuse the fluorophores into the MNPs matrix using a dye-organic solvent solution, such as NR dissolved in THF.141 This simple approach allows for incorporation of different dyes simultaneously, and provides great control over dye concentration.
Compared to stained MNPs, embedded fluorescent dyes obtained by bottom-up approaches are generally brighter and more photostable. These embedded dye MNPs are typically purchased from commercial sources and despite their advantages they suffer from a limited range of polymer types (e.g. PS, PMMA, ESI† 1) high costs and are often sold as suspension stabilized by surfactants.
A key advantage of fluorescent labels is in qualitative tracking and imaging of MNPs, for example in bioaccumulation studies. However, imaging dyes such as NR require excitation with UV light that is also absorbed by biological material, making MNP differentiation difficult. Moreover, the use of UV could damage tissues and living organisms. A possible solution to overcome this problem is the incorporation of a fluorophore requiring lower excitation energy. For example, Yakovenko et al. (2022) demonstrated top-down synthesis of MNPs embedded with lanthanide-based upconverting nanoparticles that can be excited by near-infrared radiation and emit in the visible region, facilitating benign tracking and imaging of MNP accumulation in organism tissues.142 Overall, when used appropriately, fluorescent labeling is well suited to direct identification and tracking MNPs. However, sizing of MNPs using fluorescence intensity is qualitative, and there is the potential interference from background fluorescence, risk of false positives, underestimation of polymers with weak fluorescence (e.g., PVC), possible quenching by color pigments and the need to properly select the fluorophores.143,144
3.2 Metal tagging
Tagging MNPs with metals is an alternative and in many ways complementary approach to fluorescence-based labeling. The strength of metal-tagging is the ability to detect and quantify MNPs by means of analytical techniques such as ICP-MS, as opposed to the optical imaging capabilities afforded by fluorescent tags. Given the high analytical sensitivity of techniques such as ICP-MS, metal tagged MNPs can be detected at much lower and environmentally relevant concentrations as compared to traditional detection methods such as Raman or UV-vis.41 For metal-labeled MNPs, the lower detection limit for particle size is determined by the mass of the metal. Moreover, if the metal tag is from an element with low natural environmental abundance (e.g. Pd, Ta) the MNPs can be discriminated from carbon-based background interference. The metal label itself can take many forms, such as a nanoparticle, an ion, or an organometallic compound.106,113,145 Consequently, there are a wide variety of procedures that can be employed to incorporate metal labels into different polymer types. In addition to ICP-MS, metal labeled MNPs can also be analyzed by single particle ICP-MS (spICP-MS), where each metal-labeled MNP is detected as an individual pulse in the plasma. Analysis of the pulse height distribution and frequency enables determining the size distribution as well as the number concentration of MNPs.83A common approach to preparing metal-tagged MNPs is via surface attachment of the metal tag through physical adsorption or chemical bonding. One method proposed by Jiménez-Lamana et al. (2020)106 utilized the presence of carboxylate groups at the surface of PS MPs to electrostatically attach positively charged gold nanoparticles for detection by spICP-MS. The MNP size was then determined by the generated metal signal intensity, due to the proportional relationship between the number of attached gold nanoparticles and the surface area of the MP. Despite detection of MNPs with sizes up to 1 μm in water samples, it is unlikely that this attachment strategy can be applied in more complex matrices containing other negatively charged species, such as dissolved organic matter, that will likely compete for the positively charged gold nanoparticles. Similar surface attachment strategies using the ability of MNPs to adsorb trace metal ions146 and hydrophobic organic compounds have also been employed.145 In the case of direct metal ion labeling, high concentrations of tagged MNPs must be used to generate enough metal mass for ICP-MS detection, and sorption is metal-dependent.145 Use of a hydrophobic organometallic probe (metal phthalocyanine) also enabled MNP detection by spICP-MS. However, multiple recovery steps to separate the MNPs from water-insoluble probe aggregates could lead to an underestimation of MNP concentrations.145
Unlike fluorescence staining, surface attachment of metal tags allows for application in photochemical weathering studies and quantitative analysis. However, these surface alterations could potentially modify MNP interactions and environmental behavior, much like the fluorescence staining strategies discussed above. Additionally, changes in concentration, pH, or temperature could cause desorption and a decrease in the surface concentration of the metal tag, thereby lowering detection sensitivity and limiting the potential matrices in which the MNPs could be deployed.
To avoid desorption, an alternative and more robust approach to a surface-attached metal tag is fabrication of a MNP containing an entrapped metal. In a method developed by Mitrano et al. (2019) metal-tagged MNPs were synthesized by first preparing a PAN core containing Pd followed by addition of an outer PS shell.111 This metal core–polymer shell structure allows for larger quantities of metal atoms to be contained within each MNP as compared to surface attachment, significantly improving detection and quantification by ICP-MS. The outer shell also minimizes the leaching potential of the metal tracer, thus allowing for use in ecotoxicity studies. Additionally, metals like Pd, not found at high environmental concentrations, improve selectivity in complex, heterogeneous matrices. The outer shell can also be modified to a different polymer-type for direct assessment of multiple MNP surface interactions with aquatic or terrestrial environments. For example, Rauschendorfer et al. (2021) prepared core–shell MPs containing intercalated metal chloride salts of gold, platinum, or palladium and PMMA or PS shells.112 Tracking of the unique metal signature can be used to identify reference MNPs of different polymer types. Importantly, these types of labeled reference MNPs are well-suited for fate studies in complex systems and environments, such as soils, plants, and wastewater treatment plants.
One of the disadvantages of metal core–polymer shell MNPs is that they require a somewhat complex synthesis process with multiple reaction steps and solvents. In principle, polymer shells are synthesized through emulsion polymerization.147 As a result, a number of polymers, including polyamides, polyesters (PET, PBT, PLA), polycarbonates, and silicones cannot be produced with metal cores.147 Polyolefins (PE, PP) can theoretically be polymerized by emulsion; however, this requires the application of high pressure.148 Additionally, the metal tracer cannot directly provide MNP size or shape information, thereby limiting its application in studies aiming to examine particle-size changes or size-dependent MNP properties. These core–shell syntheses also yield spherical particles of monodisperse sizes which are not representative of realistic environmental MNPs. Another disadvantage is that incorporation of the metal core modifies the density of the tagged-MP compared to an untagged-MP;112 therefore, these materials are unable to accurately probe the colloidal stability of MNPs.
An alternative to the fabrication of metal core–polymer shell MNPs is the distribution of a metal tag homogeneously throughout the polymer matrix. In a method developed by Smith et al. (2024), MNPs containing uniformly distributed organometallic additives were prepared using solution casting and cryomilling.83,149 Similar to the detection method of core–shell MNPs, metal-tagged MNPs were detectable by spICP-MS in a variety of environmental matrices at low μg L−1 concentrations. This approach is relatively straightforward and the synthetic flexibility enables the preparation of a suite of metal-tagged NPs for a variety of commercial polymers including PS, PMMA, PVC, LDPE, and PVP. In contrast to the core–shell method, the metal-tag is distributed throughout the entire volume of the microplastic and thus any changes to the size of the MNPs during an experiment can be examined. The metal-tags are present at a sufficiently low concentration that they do not modify the surface characteristics of the MNPs, in contrast to the surface bound metal-tags that rely on electrostatic attractions.
The incorporation of metals can also be combined with other labelling approaches as demonstrated by Luo et al. (2022) who entrapped a metal–luminophore complex (i.e. tris(thenoyltrifluoroacetonato)europium) in PS using an emulsion polymerization reaction.150 The resulting MNPs were metal-tagged and luminescent when UV-irradiated, which enabled not only their visualization but also their quantification.
3.3 Isotope labeling
Isotope labeling is a technique where isotopes, stable or non-stable, are incorporated into MNPs to facilitate their detection. To enable isotopically labelled MNPs to be clearly distinguished and identified in realistic environmental media and limit the potential for signal interference, the isotope concentration in the labelled MNP should be much higher than the natural abundance of the isotope found in the environment.Since stable isotope enrichment affects only the molecular weight of the polymer backbone while retaining its structural and elemental composition, labeled and non-labeled particles are expected to elute chromatographically at the same time in analytical techniques, such as Py-GC/MS or TED-GC/MS. However, since they differ in terms of mass isotopically labelled MNPs can be distinguished by mass spectrometry. In this way deuterated MNPs are frequently employed as internal standards, as they are more affordable than 13C-labeled MNPs.156–158 For example, the recovery of deuterated standards spiked before analysis with Py-GC/MS has enabled the quantification of environmental MNPs to be internally calibrated.158 However, at high temperatures, there is the potential for inorganic compounds to react with the labeled MNP and trigger hydrogen–deuterium exchanges, compromising this analytical approach.157
To-date, 13C labeled MNPs have been primarily utilized to assess the degradation rates of biodegradable macroplastics such as PBAT used in mulch films159 as well as MNPs made of more conventional, persistent polymers like PE.151,152,160 In these scenarios, the use of GC-MS isotope ratio mass spectrometry allows the tracking of 13CO2 or 13CH4 and an accurate assessment of the biotransformation kinetics.159,161 Indeed, some researchers have claimed that using 13C isotopically labeled plastics is the only reliable method to evaluate MNP biodegradation.16113C-labeled MNPs are also well-suited for fate studies, as 13C can be easily distinguished from other carbonaceous compounds, although as far as we are aware this application has never been reported. One reason for the lack of applications for fate studies could be the high prices of isotope labeled-particles, which favor the use of other labels (e.g. metals) instead. Nonetheless, stable isotope labeling is a promising tool for MNPs research because the environmentally relevant properties of isotopically labeled particles will almost always be identical to non-labeled particles, in contrast to issues that can arise with other labeling techniques.153
The detection of radioisotope-labeled MNPs can be performed by non-invasive imaging techniques such as positron emission tomography or single photon emission computed tomography techniques which measure the radiation emitted as a result of the isotope decay (positron or gamma rays).168 These instruments are sensitive to very low concentrations of isotopes with limits of detection and quantification on the order of ∼1 ng particles per g per sample.110 Furthermore, the field of view of these techniques is large, enabling imaging of large surfaces (i.e. a whole body); however, the resulting image resolution is relatively poor.168 Another advantage is that the emitted radiation can pass through matter, allowing for labeled particles to be tracked in vivo without destructive sample preparation.169 As long as the radionuclides have not decayed, samples can be reanalyzed multiple times. Conversely, the short half-life times of metallic radionuclides, including 64Cu, 131I, or 65Zn (t1/2 < 10 days), limit their use for long-term fate experiments.168 The impact of the ionizing radiation emitted by the labeled material must also be considered. For example, the radiation may induce additional toxicity, rendering radiolabeling potentially unsuitable for certain toxicity studies. Perhaps the biggest limitation of using radioisotopes is the necessity of dedicated facilities for radioactive substances,170 specialized instrumentation, and measures to protect workers. The implementation of rigorous safety protocols can impede the comprehensive characterization of labeled materials, as exemplified by Al-Sid-Cheikh et al. (2020), who could not image their 14C-labeled PS with TEM due to the aforementioned constraints.110
4 Simulation of environmental weathering
Plastics in the environment may undergo various biotic or abiotic processes that alter their properties.14,171,172 Weathering or aging is therefore a key aspect to consider when preparing environmentally-relevant MNP test and reference materials. Abiotic environmental weathering processes include oxidation, induced by sunlight or exposure to oxidants and physical transformations caused by wear or abrasion. Microbial activity can also alter MNPs through biofilm formation. To mimic the weathering processes mentioned above, pristine MNPs can be transformed, either in the laboratory or outdoors under natural conditions. Aging MNPs by exposing them to natural conditions is cheap, simple, and realistic, but usually requires long exposure times to observe significant transformations. Alternatively, laboratory-based studies allow for controlled accelerated weathering of particles and significantly reduce the exposure times compared to the equivalent natural process.14,173 In general, artificial weathering promotes uniform aging of the material, which may not be achievable in the environment due to variable weather, an important issue when the goal is to produce a well-defined reference and test material.174 However, artificial weathering can require relatively expensive equipment. Moreover, accelerated weathering may not mimic the processes taking place in the environment, resulting in differently aged particles.175 This is because various weathering mechanisms occur in the environment at the same time and these may need to be combined to produce realistic MNPs. For example, plastics found on a beach are likely to undergo both oxidation due to sunlight exposure and physical weathering caused by erosion from particles in the sea surf.Regardless of the weathering method applied, it is essential to adequately characterize the resulting particles. Alimi et al. (2022) proposed a list of properties to evaluate weathered MNPs, mainly physicochemical surface and bulk properties such as surface morphology (roughness and presence of cracks),171 with two purposes: comparing the properties of artificially and naturally aged MNPs and benchmarking studies with different materials. Furthermore, studies that compare the fate and ecotoxicological effects of artificially-aged and environmental MNPs would be useful to determine if MNPs weathered by these two means and have similar characteristics behave similarly. One the challenges to these comparative studies is that obtaining large amounts of environmental MNPs is extremely challenging as discussed in section 2.2.
The following sections provide a concise overview of the effects of weathering on the properties of MNPs and how to reproduce or simulate weathering. More detailed discussions of materials' weathering processes can be found in ref. 171, 173 and 175–177.
4.1 Oxidative weathering
Several studies have shown that, as for macroscopic plastics, exposing MNPs to oxidative conditions created either by UV irradiation from sunlight or as a result of exposure to ozone,70 can affect a wide range of their properties, including surface morphology, surface chemical composition (due to the formation of oxygen-containing functional groups such as carbonyls178), and consequently, the particle's hydrophilicity173,178 and color.16,179 Such oxidative conditions may also cause MNP fragmentation, a reduction in the molecular weight of the constituent polymers,180 as well as an increase in crystallinity which may cause material brittleness.16,171,181,182 In addition, as has been discussed previously oxidative weathering can be an unintended consequence of MNP production, particularly for top-down approaches.To date, most studies on lab-simulated environmental aging of MNPs were designed to mimic photooxidation processes.171,172 For example, filtered UV light is often applied in a temperature-controlled weathering chamber to simulate daylight conditions70,183,184 and can provide much higher radiation doses than in the environment. ISO and ASTM protocols171 have been established for testing the physicochemical properties of plastics and for accelerated weathering procedures describing how to simulate photooxidation in different environmental compartments. In brief, these protocols provide a general description of the apparatus, the parameters to monitor (i.e. relative humidity, temperature), and the methods for characterizing weathered materials. However, these protocols must be considered with care. For example, some suggest the use of xenon lamps for photooxidation, but those may produce excessive radiation in the UVC region (specifically <300 nm) and may not accurately depict the broad spectrum of sunlight.171,174
4.2 Mechanical weathering
Although the aging of MNPs due to oxidative conditions is relatively well documented, studies on physically induced weathering of MNPs are much more scarce.16,182,185,186 Historically, mechanical aging processes have been overlooked, as the chemical industry had little interest in assessing the fragmentation of plastics.16 In one example of a laboratory experiment, the effects of wind entrainment and transport in sediment revealed that physically induced weathering generated cracks on the surface of the plastics and tended to produce more rounded particles over time.185,186 Physically induced weathering can be performed with a simple setup such as sand-filled bottles on a roller mixer,182 or in a more complex manner employing a continuously aerated glass test-tube chamber.185 The application of mechanical energy affects the surface morphology of plastics and is generally viewed as the key step to fragment particles into smaller pieces, including NPs.86,185 There is clearly a need for more systematic studies of mechanical weathering and how this relates to MNP formation.1874.3 Biological weathering
While biodegradable polymers, such as PLA biodegrade quite rapidly in many environments (e.g. soils), biological transformations of MNPs made of conventional petroleum-based polymers such as PE occur over many decades in most microbial populations. As a result, MNPs from these polymer types are considered to persist indefinitely on the timescale of most laboratory studies.14,171,177 It is important to note that several ISO and ASTM protocols have been established to measure the effects of microbial activity on MNPs (e.g. CO2 production to measure biodegradation – ASTM D5988). However, despite the absence of biological transformations to the bulk of many MNPs, their surface properties may be modified due to the eco-corona coating or biofilm formation, which could significantly influence their fate or ecotoxicological effects. For example, the coating may alter the effective density and hydrophilicity of the MNPs, or favor the leaching of additives.177,178 Approaches to extract MNPs from biological media without damaging the biofilm coating and to accurately measure and quantify their transformed properties have yet to be developed due to the inherent challenges. There have been attempts to incubate MNPs to grow biofilms,171 for example in wastewater,188 microalgae culture,189 or lake water,189 to then characterize the resulting biofilm (see Alimi et al.171 for more references). Standardization of these protocols would be valuable, as coating of MNPs with biofilm or organic matter in a reproducible manner would make it easier to compare the results of studies conducted in different laboratories.5 Preparation of liquid and solid MNP dispersions
Reference and test MNPs are produced and utilized in three forms: powder, suspension, and embedded in a solid matrix. When MNPs are obtained as dry powder, they may be directly added to relevant media. However, establishing dispersion protocols is often required to spike in an experiment or to accurately mimic their form in relevant aqueous media.38 Furthermore, successful dispersion is often needed to ensure the accurate characterization of the MNP's size distribution and concentration. The dispersibility of MNP particles within a liquid medium is a reflection of the particle's colloidal stability, which in turn is influenced by MNP size, composition, and surface properties such as hydrophobicity and surface charge.190 For micron-sized MNPs, density and size also determine whether a particle will remain suspended, float, or sediment in a liquid.190 All these characteristics are crucial determinants of MNP homogeneity and stability within aqueous media.In reality, when added to water as powders, MNPs often fail to disperse and rapidly form aggregates or adhere to the walls of the vessel because of their hydrophobicity.65 To overcome this issue, ultrasonication is sometimes employed to facilitate disaggregation of individual particles and enhance the dispersion in the medium.66 In toxicology, a standard method for dispersing nanomaterials is the NanoGenoTox protocol, which involves applying ultrasound at low temperatures for 16 min at 400 W and 10% amplitude.191 Originally developed for other nanomaterials, this protocol has been adapted to generate highly stable nanoplastic solutions.192 However, ultrasonication can result in oxidation and fragmentation, as described in section 2.3.2 and so this needs to be assessed. For hydrophobic MNPs (e.g. PVC) less polar solvents such as ethanol, isopropanol or 1-propanol have proven to be effective in promoting dispersion, although solvent selection criteria must align with the research question. For example, Parker et al. (2023) selected 1-propanol over ethanol and isopropanol for suspending PVC and PP particles (0.1 wt%) due to its superior dispersion efficiency and reduced cytotoxicity.64 Alternatively, surfactants38 or biosurfactants118 can be added to water to stabilize reference MNPs and minimize agglomeration. However, surfactants modify surface properties by binding to the hydrophobic surface of MNPs, thereby rendering them hydrophilic.38 Indeed, the use of surfactants has led to numerous erroneous results regarding the properties of MNPs, such as toxicity and transport.38 To avoid the use of surfactants, Eitzen et al. (2019) enhanced the dispersion of PS particles by applying ozone (O3) to promote the oxidative modification of the surface.65 Ozone reacts with aromatic and aliphatic carbon atoms at the MNP surface and forms carbonyl, carboxyl and hydroxyl groups, thus increasing the hydrophilicity of the PS. In essence, ozonation serves as a form of accelerated weathering. As such, this process achieves the stated goal of improving MNP dispersion with the caveat that MNPs dispersed in such a fashion can no longer be classified as pristine MNPs.
Instead of being used in suspension or as a pure powder, MNPs have also on occasion been incorporated into a solid matrix. Solid dispersions are preferred as the means to prepare MNP kits containing several polymer types, and also in situations where the size or polymer type precludes the formation of stable MNP suspensions. Another advantage of these solid matrices is that the use of a physical diluent makes it easier to accurately weigh a low amount of MNPs incorporated within a larger sample mass. In one example of a solid matrix, Seghers et al. (2023) proposed freeze-drying a solution of NaCl containing MNPs to form a carrier.193 Similarly, gelatin or soda capsules have been used as solid matrices to encapsulate MNPs, selected because they dissolve easily in water and under moderate heating (40 °C) to release the particles.194 Since the capsule-to-capsule mass variability, even for particle sizes down to 50 μm, was low, the capsules could be used to perform an interlaboratory comparison of analytical methods. Another approach is where MNPs are dispersed in an inert particulate matrix (e.g. SiO2,195 alumina,196 CaCO3 (ref. 197)). Dispersing MNPs into a solid matrix is particularly important for mass-based quantification methods such as Py-GC/MS, as it facilitates the preparation of calibration standards where the MNP concentration is comparable to values expected in real-world environmental samples. Thus, as part of the development of calibration kits for Py-GC/MS where MNPs from 12 polymer types were dispersed in a solid diluent, Frontier Laboratories reported that SiO2 was suitable for the analysis of every polymer type, except PU because of uncontrolled reactions with PET in the kit during pyrolysis.195 This occurred because the pyrolysis of PET creates an acidic environment that alters the products of PU pyrolysis and consequently the reliable quantification of PU is compromised. Dispersion of PU in alumina failed to address this issue;196 however, the use of CaCO3 was found appropriate for all polymer types. This was ascribed to the ability of CaCO3 to act catalytically to transform reactive products generated during the pyrolysis of PU into stable compounds that can serve as markers for the identification of the parent PU.197 These kits are important because they can serve as the basis for inter-laboratory referencing of analytical methods used for MNP quantification. While there has been some progress in this area, the lack of MNP characterization in the matrix, particularly the size, poses a challenge.
6 MNP characteristics and their relevance in MNP research
Ensuring that reference and test MNPs are appropriately characterized before use is a critical step in improving the reliability and comparability of research outcomes. While commercially available MNPs provide a convenient starting point, their physicochemical characteristics – such as additive content, surface properties, and stability – are often unknown or poorly documented, complicating efforts to elucidate the relationships between the physicochemical properties of MNPs and their fate and interactions in environmental and biological systems. As highlighted earlier, in-house characterization is essential to verify the physicochemical properties of MNPs and assess alterations to these properties that may occur intentionally or inadvertently during their production. The following sections summarize the various characteristics that collectively describe MNPs. They are presented and ordered by the frequency with which they are reported and/or characterized in studies using reference and tests MNPs (see Table 3). These physicochemical properties of MNPs link synthesis to their applications, as the research endpoint determines the properties of interest in the produced reference and test MNPs. The aim is to describe those MNP key properties and provide relevant examples of biological effects, environmental fate, or implications for detection to illustrate the importance of characterizing specific MNP properties in different types of research studies. The most widely used analytical techniques are also introduced, without providing an exhaustive discussion of the analytical techniques themselves, which is beyond the scope of this review. A concise summary on the analytical techniques used to interrogate MNPs is provided in Fig. 5 and Table 5, while more detailed information can be found in several recent reviews on the characterization of MNP properties.41,198–202| Study type | Research question | Matrix | Reference or test MNP | Use | Characterized property (technique) | Key property | Detection technique | Ref. |
|---|---|---|---|---|---|---|---|---|
| Ecotoxicity | Impact of NPs on mortality, riboflavin secretion and iron reduction | Bacterial cell | Metal doped PS, PS (30 μm thick) and PE (25 μm thick) films (Goodfellow) | Tracer | Size (DLS, SEM, STEM-EDX), ζ (ELS) | Polymer type | ICP-MS | 203 |
| Ecotoxicity | Chemical toxicity of leachates of MPs | Bacteria, algae, larval stage of echinoderms | PET (milling) | Tracer | Polymer type (ATR-FTIR), shape (FE-SEM) | Polymer type, size | FTIR | 68 |
| Ecotoxicity | Impact of MPs on cell aggregation | Freshwater microalgae cell | UCNPs-labelled and UCNPs-free HDPE (grinding) | Tracer | Size (granulometry and NTA), shape and texture (AFM), crystallinity (DSC), chemical structure (SAXS), ζ (ELS), luminescence, hydrophobicity (FluidFM) | Hydrophobicity | Optical microscopy, AFM imaging | 204 |
| Ecotoxicity | Bioaccumulation, toxicity and transfer of NPs | Periphyton, aquatic snail | Metal doped PS (polymerization) | Tracer | Pd concentration (ICP-MP), location of Pd (TEM-EDX), leaching of Pd, size (NTA), ζ (ELS) | Size | ICP-MS | 205 |
| Ecotoxicity | Uptake and impact of NPs on mortality and growth | Gammarus pulex | Metal doped PS (polymerization) | Tracer | Location of Pd (TEM-EDX), leaching of Pd, size (DLS), shape (SEM) | Shape | ICP-MS | 206 |
| Ecotoxicity | Impact of protein corona on the regulation of NPs-induced autophagy | Mouse cell | PS (300 nm, BaseLine ChromTech Research Centre), fluorescent PS (Tianjin Da'e Technology) | Tracer | Shape (TEM) | Size, ζ, protein corona | Flow cytometry, confocal microscope, ELS | 207 |
| Ecotoxicity | Intestinal toxicity of MP fibers | Zebrafish | PP microfibers (cryomicrotomy) | Tracer | Polymer type (Raman), shape (UV-vis) | Shape | Raman, fluorescence microscopy | 208 |
| Ecotoxicity | Impact of MP fibers and particles on feeding, lipid accumulation, growth, and moulting | Crustaceans | PA granules and microfibers (Goodfellow, cryomicrotomy) | Tracer | Bulk plastic composition (GC-MS) | Shape | Fluorescence microscopy | 209 |
| Ecotoxicity | Shape-dependent accumulation and toxicity of MPs | Zebrafish | Fluorescent and pristine PP and PS (grinding) | Tracer | Polymer type (Raman), shape (fluorescence microscopy) | Shape | Raman, fluorescence microscopy | 210 |
| Ecotoxicity | Comparison of toxicity of MPs, MP fibers and NPs | Mussels | PA microfibers (PA filament, Goodfellow, microtomy), PS (20 μm, Sigma-Aldrich), and fluorescent PS (50 nm, Magsphere) | Tracer | Size and dispersion stability (TEM and DLS), ζ (ELS) | Size, shape | Optical microscopy, fluorescence spectrometry | 211 |
| Ecotoxicity | Toxicity of MPs and MP fibers | Waterflea | Fluorescent PET fiber (orange fluorescent fleece, chopping), PE (1–4 μm, Cospheric) | Tracer | Polymer type (ATR-FTIR), size (fluorescence microscopy) | Shape, size | Stereo and fluorescent microscopy | 212 |
| Ecotoxicity | Toxicity of MP fibers | Algae | Polyester (polyester monofilament fiber, Huixiang Fiber, microtomy) | Tracer | Shape (optical microscopy), length distribution (counting chamber) | Size | Optical microscopy, SEM | 213 |
| Ecotoxicity, aquatic fate | Environmental fate, ingestion and impact of NPs on physiology and structure of the organisms | Cyanobacterium, green algae, Daphnia magna | Metal doped PS (polymerization) | Tracer | Mass concentration (TOC-VCSH), size (DLS and TEM), shape (TEM), ζ (ELS) | Size | ICP-MS | 214 |
| Ecotoxicity, aquatic fate | Environmental impact and toxicity of MPs | Simulated seawater, ultrapure water, marine bacteria | PP, PS, PE, PET and PLA (dissolution–reprecipitation of conventional plastic products) | Tracer | Polymer type and surface oxidation (FTIR), size (DLS) | Polymer type, surface oxidation | FTIR | 215 |
| Ecotoxicity, human toxicity | Toxicity of NPs | Bacteria, yeast, algae, protozoans, mammalian cells, crustaceans, midge larvae | Carboxylated PS (26 and 100 nm, Bangs Laboratories) | Tracer | Size (DLS, SEM) shape (SEM), dispersion (ELS), ζ (ELS), concentration (SLS) | Additives, particle adherence on the organism | SLS | 216 |
| Ecotoxicity, human toxicity | Toxicity of MPs | Mice, human cornea and conjunctival cells | Fluorescent PS (2 μm and 50 nm, Baseline ChromTech Research Center and Beijing Dk Nano Technology) | Tracer | Size, shape (TEM), polymer type (FTIR) | Size | Fluorescence microscopy | 217 |
| Ecotoxicity, human toxicity | Effect of inhalable MP fibers on lung | Human and murine lung organoids | PET and PA microfibers (Goodfellow and commercially available fabrics, cryomicrotomy) | Tracer | Concentration, shape and size (SEM), elemental composition (EDX), polymer type (FTIR) | Polymer type, leachate | — | 218 |
| Human toxicity | Impact of gastrointestinal digestion and colonic fermentation on biodegradable MPs | Gastrointestinal digestion fluid, human colonic microbiota | PLA (milling) | Tracer | Shape and texture (FESEM), crystallinity (Raman) | Crystallinity, polymer type | FESEM | 60 |
| Human toxicity | Polymer dependent toxicity of NPs | Human cells | PET, PVC, PS (solvent removal) | Tracer | Concentration (gravimetry), shape (SEM), size (DLS), ζ (ELS), polymer type (FTIR) | Concentration, exposure time, polymer type, density, hydrophobicity | Confocal microscopy | 219 |
| Human toxicity | Toxicity of MPs | Human cells | ABS, PVC (ball milling) | Tracer | Size, shape, texture (SEM and optical microscopy) | Polymer type, shape, texture | — | 220 |
| Human toxicity | Toxicity of MPs | Human cells | HDPE (1–10, 50 and 100 μm, Cospheric), LDPE (cryomilling) | Tracer | Size, shape, texture (SEM), polymer type (Raman) | Concentration, shape, texture | — | 221 |
| Human toxicity | Toxicity of NPs used in food packaging/beverage bottles production | Intestinal epithelial barrier and liver cells | PC and PET (laser ablation) | Tracer | Size (DLS), surface chemistry (XPS), shape (TEM) | Size, polymer type and surface chemistry | — | 77 |
| Human toxicity | Uptake and toxicity of NPs | Human hematopoietic cell | Fluorescent metal doped PET (dissolution–reprecipitation) | Tracer | Size and shape (TEM), Ti content (EDX and MS), polymer type (FTIR), size (DLS), ζ (ELS) | Polymer type, size and shape | Confocal microscopy | 222 |
| Human toxicity, uptake | Toxicity of MPs of different polymer type, size and concentration | Human cells | PS (50 nm, 200 nm and 1.1 μm, Polysciences), fluorescent PS (44 nm, 190 nm and 1.04 μm, Polysciences), aminated fluorescent PS (55 nm, Magsphere) and PMMA (70 nm, 400 nm and 1.1 μm, Polysciences) | Tracer | Size (DLS), ζ (ELS) | Size, surface charge, shape | Confocal microscopy | 223 |
| Human toxicity, uptake | Uptake, distribution and toxicity of MNPs | Human small intestinal epithelium model | PS (25 and 100 nm, Phosphorex) | Tracer | Size (SMPS and APS) | Size | Confocal microscopy | 224 |
| Human toxicity, uptake | Uptake and toxicity of NPs | Human intestinal epithelial cells | PET (laser ablation) | Tracer | Shape (TEM), size (DLS), ζ (ELS), surface chemistry (XPS), dispersion (ELS) | Size | Flow cytometry, confocal microscopy | 78 |
| Human toxicity, uptake | Uptake and toxicity of NPs | Human macrophage and epithelial cells | Fluorescent functionalized PS (polymerization) | Tracer | Size and shape (TEM, SEM, DLS), ζ (ELS) | ζ | Confocal microscopy | 225 |
| Uptake | Uptake, distribution, accumulation and elimination of NP | Rainbow trout | Metal doped PS (polymerization) | Tracer | Pd concentration (ICP-MS), size (NTA) | Size | ICP-MS | 226 |
| Uptake | Uptake, distribution, accumulation and elimination | Oyster | Metal doped PS (polymerization) | Tracer | Size (TEM and DLS), ζ (ELS) | Size | ICP-MS, TEM | 227 |
| Uptake | Uptake and elimination of MPs | Oyster, mussel | Pristine (113, 287, 510, and 1000 μm), and fluorescent (19 μm) PS (Polysciences and Cospheric), PA fibers (A.C. Moore) | Quality control | Size and shape (optical microscopy), polymer type (Raman, FTIR) | Size, aggregation | Fluorescence microscopy | 228 |
| Uptake | Uptake, effect of surface charge and wettability | Oyster, mussel | Pristine and carboxylated fluorescent PS (10 μm, Polysciences) | Tracer | ζ (ELS), hydrophobicity (contact angle) | ζ, hydrophobicity | Flow cytometry | 229 |
| Uptake | Uptake, accumulation and elimination of NPs and MP fibers | Soil, earthworm | Metal doped PS (polymerization) and metal doped PET fibers (extrusion and cutting) | Tracer | Pd concentration (ICP-MS), size (DLS) | Size, shape | ICP-MS | 230 |
| Uptake, terrestrial fate | Transport of NPs in soil | Soil, earthworms | Metal doped PS (polymerization) | Tracer | Pd concentration (ICP-MS), size (DLS) and ζ (ELS) | Size, shape | ICP-MS | 231 |
| Terrestrial fate | Transport of NPs and MP fibers | Simulated soil (unsaturated porous media) | Metal doped PAN and metal doped PS (polymerization) | Tracer | Pd concentration (ICP-MS), size (DLS), shape (SEM, STEM-EDX) | Size, aggregation | ICP-MS | 232 |
| Terrestrial fate | Transport of NP | Saturated natural porous media | Pristine, carboxylated and aminated fluorescent PS (191 nm, 114 nm and 51 nm; 52 nm; 57 nm, Magsphere) | Tracer | ζ (ELS), size (DLS) | Size, surface chemistry, ζ | Fluorescence spectrophotometry | 233 |
| Terrestrial fate | Plant uptake and translocation of NPs | Wheat, lettuce | Lanthanide fluorescent chelates doped PS (polymerization) | Tracer | Size (DLS), shape (STEM), elemental composition (EDX), ζ (ELS), stability and leaching of tracer (fluorescent spectroscopy), concentration (ICP-MS) | Size, shape, aggregation, ζ | ICP-MS, fluorescent spectroscopy, SEM | 150 |
| Aquatic fate | Fate and physicochemical modification of NPs in drinking water treatment process | Deionized and lake water | Metal doped PS (polymerization) | Tracer | Size (DLS), ζ (ELS), Pd distribution (STEM), Pd concentration (ICP-MS) | Size, ζ | ICP-MS | 234 |
| Aquatic fate | NPs depolymerization | Water | PET (dissolution–reprecipitation) | Tracer and quality control | Shape (FE-SEM), size (DLS) | Polymer type | HPLC | 122 |
| Aquatic fate | Fate of MP fibers | Sludge | Metal doped PET fibers (extrusion and cutting of PET granules, Serge Ferrari Tersuisse Multifils) | Tracer | Shape and size (SEM, optical microscopy), In concentration (ICP-MS) | Size, shape, concentration, density, polymer type | ICP-MS | 98 |
| Aquatic fate | NPs and MP fibers flux | Sludge and effluent from a pilot WWTP | Metal doped PET (polymerization) | Tracer | Size (DLS and optical microscopy), ζ (DLS), shape (SEM), In distribution (STEM-EDX), In concentration (ICP-MS) solid content of the stock dispersion (TGA) | Aggregation | ICP-MS | 235 |
| Aquatic fate | Degradation of MPs | Seawater | PET and PA fibers (Helly Hansen and Pierre Robert Group, microtomy) | Tracer | Shape and size (optical microscopy and SEM) | Size, polymer type, additives | SEM, GC-MS and LC-UV/LC-MS/MS | 236 |
| Aquatic fate | Environmental degradation of MPs including additives leaching | Seawater, freshwater | PET (Helly Hansen), PAN (Varner) and PA (PRG) microfibers (microtomy) | Tracer | Polymer type (ATR-FTIR), size and shape (optical microscopy and SEM) | Polymer type, matrix salinity, additives | GC-MS and UPLC-MS/MS | 237 |
| Aquatic fate, atmospheric fate | Impact of aging on chemical structure, surface shape and crystallinity of MPs | Water, air | LDPE (≤300 nm), PET (≤300 nm) and PVC (≤250 nm) (Goodfellow) | Tracer | Size distribution (Coulter counter), size (SEM) | Polymer type, shape, crystallinity | Raman and ATR-FTIR, SEM, XRD | 238 |
| Aquatic fate, atmospheric fate | Aerosolization of MNP via sea spray | Water, air | Fluorescent PS (0.5, 2, 10 μm, Polysciences), PE (1–10 μm, Cospheric) | Tracer | Concentration and size (fluorescence microscopy, Raman) | Size, density, concentration | Fluorescence microscopy, Raman | 239 |
| Atmospheric fate | Impact of shape in long-range transport of airborne MP fibers | Air | Acrylic resin fibers (3D printing) | Tracer | Size and shape (laser microscopy) | Size, shape | High speed camera | 129 |
| Monitoring | Detection of MPs | Water | PS, PET, PMMA, PVC, PTFE, PE and PMMA (Sigma-Aldrich and plastic objects grinding) | Tracer | Concentration, size and shape (confocal fluorescence microscopy) | Size | Confocal fluorescence microscopy | 240 |
| Monitoring | Identification of single NP particles and agglomerates | Water | PS (800, 300 and 100 nm, Sigma-Aldrich) | Tracer | Polymer type (SERS) | Size | SERS | 241 |
| Monitoring | Quantification of NPs | Tap, mineral, and river water | PS (100, 300, 600 and 800 nm, Sigma-Aldrich) | Tracer | Texture and thickness (AFM) | Size | SERS | 242 |
| Monitoring | Detection and identification of NP | Milli-Q and river water | PS (600–1000 nm, Aladdin and 5 μm, ChromTechResearch Center) | Quality control | Size (DLS), concentration (NTA, UV-vis) | Size, shape, density | SEM | 243 |
| Monitoring | Identification of NPs | Commercially bottled drinking water | PS (50 nm, Phosphorex, 200, 500 and 1000 nm, Sigma-Aldrich), PET (dissolution–reprecipitation and sonication) | Tracer | Surface and shape (AFM), size (NTA) | Size | SERS | 125 |
| Monitoring | Detection and quantification of NPs | Deionized, bottled, tap and river water | PS (Zhongkeleiming Technology) | Quality control | Polymer type (Raman) | Size | Raman | 244 |
| Monitoring | Detection and quantification of NPs | Drinking, tap, and river water | PS (polymerization) | Positive control | Size (SEM), polydispersity, ζ (ELS), Au label (SEM) | Size, surface charge | ICP-MS | 106 |
| Monitoring | Detection and quantification of NPs | Water, radish, mussel | PS (100 and 300 nm, Sigma-Aldrich), pristine and carboxylated PS (49 nm, Polysciences) | Tracer | Size, dispersion (DLS) | Size, surface functionalization | Fluorescence spectroscopy | 245 |
| Monitoring | Impact of MPs on gut microbiota and polymer degradation | Gastrointestinal digestion fluids | PET (cryomilling) | Tracer | Shape (FESEM), structure, crystallinity and polymer type (Raman) | Crystallinity, surface properties | FESEM, Raman | 59 |
| Monitoring | Extraction of MPs | Soil | Metal doped PET (milling) and PET fibers (extrusion and cutting) | Quality control | Metal concentration (ICP-MS), size, shape, staining (microscopy) | Polymer type, size | FTIR-ATR, microscopy, ICP-MS | 246 |
| Monitoring | Fabrication of nylon NPs | Mammalian cells | PA (dissolution–reprecipitation) | Tracer | Fluorescence (fluorescence spectroscopy), polymer type (FTIR), size and shape (DLS and FE-SEM) | Shape | Fluorescence microscopy | 247 |
| Monitoring | Identification and quantification of MPs | Human sputum | PS and mix of PE, PP, PC, PA6, PET, PU, PVC, PS, PMMA (Shanghai Microspectrum Company) | Quality control | Polymer type (LDIR) | Polymer type | LDIR | 248 |
| Monitoring | Detection of NPs | Lab equipment, hot water-contained commercial paper cup, coastal seawater | PS (300 nm, Samchun Chemicals and 200 nm, Polysciences), PMMA | Quality control | Polymer type (SERS), size and shape (FE-SEM), size of synthesized PMMA (TEM) | Polymer type, size, dispersion | SERS | 61 |
| Monitoring | Extraction and quantification of NPs | Soil, sediment | PS and PMMA (50, 100 and 500 nm, Janus New-Materials) | Quality control | Composition (Py-GC/MS and ICP-MS), shape (TEM) | Size | Py-GC/MS | 249 |
| Monitoring | Quantification of NPs | Plants | PE and PMMA (120 and 100 nm, China Petrochemical Corporation) | Quality control | Size distribution (laser diffraction), polymer type (FTIR), shape (SEM) | Thermal properties, size | Microcombustion calorimetry | 250 |
| Monitoring | Extraction, detection and quantification of NPs | Influent and effluent from WWTP, river water | PS (50, 100 nm, NIST and 500 nm, Janus New-Materials), PMMA (50, 100 nm, Phosphorex, Hopkinton and 500 nm, Janus New-Materials) | Quality control | Polymer type (Py-GC/MS), size (DLS and SEM), shape (TEM) | Size, polymer type, protein corona | Py-GC/MS | 251 |
| Monitoring | Identification and quantification of MNPs | Water, river water | PVC, PP (Sigma-Aldrich) and PS (900 mm, Goodfellow) | Quality control | Polymer type (Py-GC/MS) | Size | Py-GC/MS | 252 |
| Monitoring | Detection and identification of MPs | Tap water | PA (1–315 μm. Carl Roth GmbH), PE, PMMA (1–315 μm, 15–150 μm. Sigma-Aldrich), PP, PS (150 μm, 106–125 μm, Polysciences) | Quality control | Polymer type, size and shape (Raman) | Size, dispersion | Raman | 253 |
| Monitoring | Identification of MPs | Sediment, nail polish | PA, PET, PS, PP, PE (milling) | Quality control | Polymer type (Raman) | Size | Raman | 254 |
| Monitoring | Identification and quantification of MPs (interlaboratory study) | Deionized water | PE, PS, PVC and PET (various size ranges, NIVA and Cospheric) | Quality control | Color, shape, concentration (optical microscopy) | Size, shape, polymer type, color | Optical microscopy, FTIR, Raman | 255 |
| Monitoring | Extraction, identification and quantification of MPs | Seafood | PS, PMMA, PVC (Sigma Aldrich), LDPE (500 μm. ThermoFisher Scientific), PET (20–110 μm. NIVA) and PP (20–120 μm. Lyondellbasell) | Quality control | Polymer type (Py-GC/MS), solubility in DCM (Py-GC/MS) | Solubility | Py-GC/MS | 256 |
| Monitoring | Identification and quantification of NPs | Surface water, groundwater | PS (200 nm, Zhongkeleming Technology), PVC, PMMA, PP, PS, PE, PET (Macklin Biochemical) | Quality control | Size (DLS), polymer type (Py-GC/MS) | Size | Py-GC/MS | 257 |
| Monitoring | Extraction of MPs | Fishmeal | PET, PVC, HDPE, LDPE, PP, PS (commercial products, grinding and cutting) | Quality control | Polymer type (ATR-FTIR) | Color, density | Optical microscope | 258 |
| Monitoring | Quantification of MPs | Wastewater influent | HDPE, PP, PS (LG Chem), PET (Lotte Chemical), PA (Hyosung TNC), HDPE, PS, PET (20–100 μm. Korea Testing and Research Institute) | Quality control | Size (optical microscopy and DLS), shape and texture (FE-SEM), density (density gradient) | Size, mass, polymer type | FTIR, Py-GC/MS | 259 |
| Monitoring | Detection of MPs | Wastewater, sludge | PS, LDPE (Ineos), PET (TPL), PLA (Nature Works), PA (Lanxess), PMMA (<125 μm Röhm), PS (40 μm, BS-Partikel) | Quality control | Size (DLS), staining (fluorescence microscopy) | Size, stability of dispersion, NR staining efficiency and robustness | Fluorescence microscopy, FTIR | 260 |
| Monitoring | Quantification of MPs | Blood | PMMA (Cospheric), PP (Sigma-Aldrich), PS (Cospheric), PE (Cospheric), PET (Goodfellow) | Quality control | Polymer type (Py-GC/MS) | Size, polymer type | Py-GC/MS | 22 |
| Monitoring | Extraction of MPs | Wastewater effluents | LDPE, PVC, PS, PP, PET, PLA and PA (sonication) | Quality control | Size (fluorescence microscopy) | Size | Fluorescence microscopy | 67 |
6.1 Polymer type
While polymer type is often the baseline characteristic used to describe MNPs, it is usually not a stand-alone predictor of MNP properties and needs to be considered alongside other MNP properties such as size and shape. Moreover, although polymer type defines the surface properties of pristine MNPs', they can be altered, sometimes significantly, by weathering. In some instances, however, differences in polymer type may provide a logical explanation for MNP behavior. For example, Ma et al. (2024) observed a higher toxicity to human cells of pristine PET and PVC NPs compared with PS NPs,219 although each of the three polymer NPs had a similar size, shape and surface charge. They hypothesized that this polymer type dependent effect was due to the variations in chemical structure and hydrophobicity which impacted the nature of the adsorbed species and the resultant NP toxicity. It should be noted that knowledge of the polymer type can provide insights into the likely sources, distribution, and entry pathways of MNPs into the environment.261 For example, Deng et al. were able to clearly link the high concentration of PET fibers in water and sediment to a nearby textile industry.262 Identifying the polymer type of MNPs (e.g., FTIR, Raman, or Py-GC/MS) typically relies on matching the measured spectra with the characteristic spectra of known samples, either included in commercial libraries or collected using in-house produced reference MNPs. However, this approach is not always straightforward because polymers can contain more than one polymer type as well as other components (see 6.5 Bulk MNP composition).6.2 Size
Particle size has well documented effects on MNP fate, bioavailability, and impacts on the environment and living organisms.263,264 Indeed, MNP size is a key parameter in determining the behavior of MNPs in any study type. For example, Ward et al. (2019), demonstrated that bivalves such as oysters and mussels selectively ingest smaller particles while rejecting larger ones in the size range from 19–1000 μm, indicating a size cut-off for ingestion.228 Smaller sized MNPs were also preferentially taken up by earthworms, demonstrating the general importance of MNP size for uptake and distribution.230 In addition to preferential uptake, MNPs smaller than approximately 1 μm can cross biological barriers265 and accumulate in tissues,266 although aggregation may decrease NP toxicity by limiting bioavailability and reducing the exposed surface area.267 This size dependent behavior highlights the need to determine MNP dispersion characteristics in uptake and toxicity studies, as discussed in section 5 Preparation of liquid and solid MNP dispersions. Environmental fate of MNPs is also determined by the size; for example, Shaniv et al. (2021) showed that PS particles of 50 nm presented higher mobility in soil compared to 110 nm PS,233 while at the air–water interface, Harb et al. (2023) reported that 0.5 and 2 μm PS particles are aerosolized two orders of magnitude more easily by sea spray than 10 μm particles.239 These observations demonstrate that smaller sized MNPs have increased mobility and will transport further in the environment.The size of MNPs also needs to be known to ensure it falls within the analytical window of the detection method selected for the study.255,268 Similarly, when a new method is validated through spike-recovery experiments, the range of reference MNPs sizes for which the method has been validated should be reported, as recovery rates are also size-dependent. Smaller-sized MNPs pose particular challenges as it has been shown that detection errors increase as particle size decreases.269 In addition, Le Juge et al. (2023) has shown that the quantification of NPs by Py-GC/MS may be size-dependent.270
The importance of MNP size is reflected in it being ubiquitously reported in the studies described in Table 3. For spherical MNPs or MNP fragments generated by top-down methods, the size of an individual particle is measured by its (effective) diameter, while the size characterization of the particle ensemble should be reported in terms of the overall size distribution. For fibers, the dimensions should be reported in terms of the distributions in both length and diameter, as the aspect ratio is a critical parameter as discussed in section 6.3. Particle size and particle size distribution can be evaluated by (electron or optical) microscopy271 or by electrophoretic methods, which measure the hydrodynamic diameter.272 The most common method for the characterization of micro/nanopowders is SEM, while for dispersed nanoparticles dynamic light scattering (DLS) is by far the most preferred technique.223,243 However, DLS struggles with polydisperse samples. Nanoparticle tracking analysis (NTA) is an alternative to DLS for polydisperse MNP suspensions that analyzes particles individually. For metal-doped MNPs, ICP-MS and spICP-MS has proven to be an effective means of determining both particle size concentration and particle size distribution.273
6.3 Shape and aspect ratio
MNP shape, including aspect ratio, can also be a significant factor in controlling MNP properties and environmentally relevant behavior. Keller et al. (2020) have demonstrated that the mobility of spherical MNPs and organic solids is similar, whereas MNP fibers exhibit less mobility, emphasizing the role of shape in environmental particle dynamics and transport in terrestrial media.232 Similarly, shape is also recognized as a key parameter in the transport of MPs in water274 and the atmosphere.129,275 There are some conflicting results regarding the uptake of fibers by marine organisms compared to lower aspect ratio particles, although it is clear that shape plays an important role in the ingestion of MNPs.221,228,230 In a related MNP accumulation study, shape was found to play a role in the time required for MNPs to be expelled from the bivalves' gut, with ingested fibers being retained longer in the gut compared to spherical particles.210,228Interestingly, in a study about the impact of PE spherical beads and PET fibers on freshwater zooplankton, Ziajahromi et al. (2017) observed uptake of spherical beads but not fibers, although the external physical impact (carapace and antenna damage) caused by the fibers made them even more toxic to daphnia than the spherical beads.212 In a different study, Han et al. (2020) were able to link the shape and sharpness, as defined by a 2D-local curvature equation, of PVC and ABS resulting from milling with their toxicity to human cells.220 The fragments were not sharp enough to induce physical damage to cell wall but did trigger immunotoxicity. However, the accumulation or toxicity effects of MNPs on marine isopods were independent of the shape of MNPs (beads, fragments or fibers), suggesting that marine isopods might be less sensitive than other marine invertebrate species.276 In contrast, a study with PE MNPs supported the idea that irregular shapes help to trigger cytotoxicity and inflammatory responses, in marked contrast to the lack of toxicity observed for smooth spheres.221 Other studies have reported that the length and rigidity of fibers has also been reported to affect their toxicity;213,277,278 however, considering the contradictory results on the importance of length (i.e. longer fibers leading either to more or less damage than shorter ones), this parameter alone is not a sufficient descriptor of a fibers' toxicity, and rigidity is difficult to assess.
It should be noted, however, that there is a lack of studies where a direct comparison of shape effects on MNP's behavior is the central focus. Moreover, the overwhelming majority of studies focus on spherical MNPs or MNP fragments generated by top-down methods. An analysis of the literature highlights the importance of conducting more studies using fibrous reference MNPs. Indeed, fibers are reported to be the most or second most prominent shape of MNPs in terrestrial environments, water bodies and biota.279–282 For both characterization and detection, shape is most readily determined by using either optical or electron microscopes, depending on the MNP size.
6.4 Surface properties
Surface properties such as charge, functionalization, oxidation state, texture, adsorbed chemical or biological contaminants, etc. represent another key characteristic that define MNPs. Since surface properties are often affected by the production and labelling method (see MNP production section), for many study types it is crucial that they are well characterized to avoid data misinterpretation. Surface properties can also be altered by ageing (e.g. by irradiation) or by protocols used to extract MNPs from complex matrices, as they may require corrosive chemicals (e.g. H2O2). Further, changes in the MNP surface may impact their reliable identification and quantification. In this context, reference MNPs have been used to assess the impact that modifications to particle surfaces caused by weathering or extraction protocols, can have on the misidentification of MNPs by techniques such as FTIR, mass spectrometry, or chromatography.67 For example, Philipp et al. (2022) observed that PLA turned from transparent to milky and became more brittle after a one-day Fenton reaction.51 Lusher et al. (2017) provides a compilation of chemical resistance of seven individual polymers to different solvents, acids, and bases.283 More importantly, MNP surface properties profoundly influence their interactions with solvents and biological media, contaminants, natural particles, and organisms, which has a direct effect on processes such as uptake, transport, adsorption and biofilm or eco-corona formation.41,177,284Using zeta-potential (ζ) as a proxy, surface charge is the most widely reported surface characteristic. Notably, it was identified as an important MNP property in determining cellular uptake.285 In fact, cell membranes, which are negatively charged, have a stronger interaction with positively charged particles, which are thus more easily internalized than negatively charged MNPs. Silva et al. (2014) proposed using the magnitude of the difference in ζ between MNPs and cells or organisms as a metric of toxicity for charged MNPs,286 although the influence of other properties like aggregation and biofilm formation cannot be ignored.225 In addition, Rosa et al. (2013) reported that both oysters and mussels exhibited selective feeding, preferentially ingesting or rejecting MNP particles based on their surface charge and hydrophilicity.287 Similarly, the mobility of PS NP tracers in soil was found to be associated with their surface functional groups (among carboxyl, hydroxyl, and amino groups) as well as the resulting charge and hydrophobicity/hydrophilicity.233 Indeed, the surface properties of MNPs will influence their tendency to undergo heteroaggregation with other particulate species as well as their propensity to adsorb organic and inorganic chemicals onto their surfaces.190,288,289 Thus, Rowenczyk et al. (2020) demonstrated that the surface oxidation of plastic debris greatly influences the sorption of organic pollutants and metals, likely due to the alteration of the surface charge and hydrophobicity.290 Variabilities in surface properties could also rationalize studies that showed nominally identical particles sourced from different manufacturers which exhibit significantly different toxicity towards cells.45,291
Surface morphology is sometimes evaluated, and in a few studies it has been observed to impact MNP behavior. Thus, rougher surfaces have been seen to be more prone to pollutant adsorption or trapping292,293 due to their higher surface area-to-volume ratios; similarly, rougher surfaces also favor biofilm growth and the formation of an eco-corona which can alter the surface properties and density of the particles.177,294
The MNP surface properties that play important roles in governing MNP interactions with the environment can be characterized by various techniques. For example, XPS can provide information about the surface chemistry and composition of MNPs, while ELS is used to determine zeta potential, a proxy for MNPs surface charge.295 Surface texture is best examined by microscopic techniques such as SEM or TEM. One characteristic that has sometimes been cited as playing a central role in determining MNP properties is hydrophobicity/hydrophobicity,290 although no analytical method has emerged as being able to reliably and accurately quantify this parameter for nanoparticles.296 Finally, it should be mentioned that MNP test materials should be screened for surface contamination before use, and particularly for microbial/endotoxin contamination when biological effects are under evaluation. In this regard, typical thermal, chemical or physical treatments used to ensure particles sterility may damage MNPs, so clean production and handling, when possible, is highly desirable. MNPs can be analyzed for endotoxin contamination using limulus amebocyte lysate (LAL) test119,126 or HEK toll-like receptor (TLR) reporter cells.297,298
6.5 Bulk MNP composition
MNPs sometimes consist of more than just a single polymer. For example, plastics used to produce reference MNPs by a top-down approach may be co-polymers or polymer blends (i.e. a mixture of many polymers),299,300 or contain different types of additives.31 This type of species includes not only intentionally added substances, principally organic chemicals, but also inert elements such as metals or carbon fibers,84 added to modify certain polymer characteristics (e.g. resistance to UV aging) and occasionally even to identify the manufacturer. Indeed, plastics contain over 300 different additives, known as PoTSs (potentially toxic substances), which are typically not bound to the polymer matrix and can thus migrate to the surface and subsequently desorb, or leach from the plastic and produce toxic effects.301–303 Moreover, the adsorption of pollutants onto the MNP surface may be altered by the presence of these additives.288 The importance of additive leaching also increases as the surface area to volume ratio of the MNPs increases, so smaller particles are likely to pose increased risks in this respect. Some additives will improve the photostability of MNPs, while other additives cause color changes which may influence visual identification or the uptake of MNPs by living organisms, if they confuse the particles with food: according to de Sá et al., a higher amount of white PE than red and blue PE was ingested by fish due to its similarity with their main natural preys.304 Unfortunately, the details of the composition and concentration of these various additives in MNPs is often proprietary, although their presence has the potential to profoundly impact MNP behavior, particularly toxicity. Indeed, the impact of MNP composition on behavior is typically overlooked, likely because the challenges in determining additive speciation and concentration are considerable.Details on the MNP components and their concentration can be obtained by chromatography- and MS-based techniques such as ICP-MS, HPLC or Py-GC/MS.305 To differentiate between the effects of additives/contaminants and the particles themselves in determining the impact of MNPs, most notably in toxicity studies, it is necessary to determine additive leaching rates from MNPs generated from bulk polymers in top-down methods to avoid misinterpretation of toxicity results.306 In contrast, MNPs generated by bottom-up methods typically use polymer feedstocks that are relatively well characterized and free of additives, since these are incorporated during the manufacture of macroscopic plastics/polymers, but the absence of chemicals residual from the synthesis, particularly surfactants, must be verified.
6.6 Bulk MNP physical properties
Characterization of MNPs' bulk physical properties such as crystallinity or density, is missing in most studies reported in the literature, but these properties are important to explain certain findings related to MNP transformations, fate and toxicity.For example, crystallinity influences a plastics' mechanical properties. A higher crystallinity is correlated with strength, but also with brittleness, and can thus be considered a proxy for the likelihood of fragmentation. While crystallinity itself may have a limited impact in many MNP-related studies, its characterization is important in understanding how MNPs become embrittled and fragment in the environment, as a consequence of chemical (e.g. photooxidation) or physical (e.g. erosion) weathering, and thus change in size and shape.16 Embrittled MNPs are also more likely to fragment inside the human body when they encounter chemical and/or physical stress, such as those that occur during digestion.59 Crystallinity is also an important factor regarding the biodegradability and depolymerization of MNPs.59,60 Crystalline particles are less easily broken down by enzymes than amorphous ones, and, therefore, the polymer is less bioavailable for microorganisms.307
Density depends on the composition, molecular weight, and degree of crosslinking, and is positively correlated with crystallinity.308 Consequently, a weathered, more crystalline plastic is denser than its pristine counterpart, although the difference is typically small. Density can influence MNP fate in the environment,16 because it controls the settling velocities of particles in a fluid, and is typically calculated with Stokes' law.190 These rates moderate the transport and distribution of MNPs in water or the atmosphere,16 and therefore influence, for example, which aquatic organisms come into contact with the MNPs. However, ingestion experiments performed with MNPs of various densities suggest that density may be a negligible parameter for particle selection by marine organisms like oysters and mussels in comparison with other MNP characteristics, such as surface properties.287
MNP crystallinity is typically determined by DSC,204 whereas MNP density is rarely characterized (Table 3). This may be because density is typically not considered relevant, or that referring to densities provided in polymer textbooks or by the manufacturer is considered sufficient. This lack of reporting is also a consequence of the difficulty in measuring MNP density, although it can be estimated by determining the buoyancy of MNPs in a range of solutions with different densities.309
6.7 Concentration and dose
The effects of MNPs often depend on the concentration and/or dose, and these parameters are usually reported across all study types, although the way in which they are reported varies considerably. If the MNPs are obtained from commercial sources as a dispersion in a liquid or solid matrix, then the concentrations are almost always provided by the vendor. However, in experimental studies these values need to be checked to ensure that they have not been influenced by how the MNPs were dosed or by matrix effects (e.g. MNP aggregation). MNP concentration can also be reported in terms of particle number concentration (PNC). For polydispersed MNPs, particle size distribution (PSD) is another extremely important parameter that should be measured. Optical microscopies, micro-FTIR and micro-Raman can determine PNC and PSD for MPs, due to their larger size. In contrast, laser-based techniques310 such as dynamic light scattering (DLS) can measure PSD for both MPs and NPs, while nanoparticle tracking analysis (NTA) can determine PSD and PNC for NPs. For metal-doped MNPs, ICP and sp-ICPMS have been used effectively to determine PNC and PSD at extremely low, environmentally relevant MNP concentrations.83,232 Although electron microscopy (EM) techniques such as SEM and TEM are capable of measuring PSDs at micro- and nanoscale dimensions, respectively, these are low-throughput techniques and drying effects during sample preparation may cause unwanted particle aggregation leading to PSD distortion. Polymer mass concentration can be determined by mass spectrometry (e.g. Py-GC/MS, TED-GC/MS),311–313 and if the MNP concentration is high enough, gravimetric methods are also an option. However, in the absence of PSD information mass concentration is an ambiguous metric because a given gravimetric amount of MNP can be associated with a small or large number of MNP particles.There are a number of different scenarios where it is important to know the concentration/dose of reference or test MNPs; for example, as internal standards (e.g. in Py-GC/MS) to correct for matrix effects and/or instrumental drifts, or in method validation MNP dose/concentration should be quantified to determine recovery rates. For MNPs used to evaluate toxicological effects it is important to determine not only concentration, but also exposure time, because toxicity can be dose and concentration-dependent.77,314 Moreover, the PSD is a critical piece of information for interpreting toxicity data because it can be used to estimate the exposed surface area of MNPs that will come into contact with organisms in the environment.
6.8 MNP solution chemistry
In addition to the profound influence that electrolyte composition plays in determining MNP stability and aggregation state, any MNPs in suspension will constantly interact with other possible components (e.g. antimicrobial agents or surfactants). Thus, the composition of MNP-containing solutions or solid matrices should be detailed. In addition to the typically unwanted and confounding effects of surfactants, the potential presence of deliberately included biocides should also be considered in commercial NP suspensions.315,316 For example, Heinlaan et al. (2020) observed that the toxicity of commercially available PS NP was caused by the antimicrobial additive sodium azide and not by the particles themselves, emphasizing the need for careful consideration of additional compounds in toxicity assessments.216 In the case of bottom-up MNP production, it must be determined if surfactants, unreacted monomers, and residual polymerization by-products, including initiators, catalysts and solvents, have inadvertently remained in the final solution.16,31,41,317,3187 MNP detection
In many studies, it is necessary to detect and characterize MNPs after they have been introduced into a matrix or subjected to various treatments, and this can be challenging. The literature provides a number of examples on the detection and quantification of MNPs across diverse matrices ranging from marine organisms,319 human23,24 and animal tissues,320 air,321 water,322 dust,323 and soil.324 The selection of suitable analytical techniques for MNP detection and characterization post-experiment depends on several factors, including particle size range and concentration (which must be above the detection limits), matrix, label, state of the MNPs (powder, liquid suspension, or dispersion in an organic matrix), and the key properties to be examined (Table 5). After extraction from aqueous media or air filters, spectroscopic techniques such as FTIR and Raman as well as mass spectrometry techniques like Py-GC/MS can be used to detect MPs, although quantification of NPs is more challenging due to their smaller size. In biological media, detecting MNPs is essential for understanding cellular uptake and transport mechanisms; this can be accomplished with electron microscopy techniques like TEM, evaluating samples in vitro that have been fixed, or by in vivo analysis of pre-treated models.325–327Labelled MNPs are often used to facilitate the detection and localization of MNPs using methods like fluorescence microscopy, flow cytometry, or photoluminescence spectroscopy,149,328 without the difficulties inherent in MNP extraction. However, the label can modify the particle's surface properties and bias the results of the study.329 This has led to an increase in label-free, and rapid detection methods for MNPs.200,245,330–332
8 Application of reference and test MNPs
The previous sections detailed existing methods to produce, characterize, and detect the reference and test MNPs that are indispensable as tracers, to study their behavior and effects in controlled experiments, and as calibration and internal standards, to develop and validate analytical methods to detect and quantify MNPs. Table 3 compiles information on the various applications of reference and test MNPs retrieved from 72 research papers published between June 2013 and February 2024 identified by in the Web of Science core collection, Scopus database and Google scholar by using “microplastic*”, “nanoplastic*”, “characterization”, “reference”, “standard”, “material*”, “tracer”, “control”, “internal standard”, “labelling”, “test”, “impact”, “polymer type”, “size”, “shape”, “detection”, “quantification”, “spik*” keywords. Entries are organized according to study type, divided into toxicity (both human toxicity and ecotoxicity), uptake by organisms (entry and distribution), environmental fate (including terrestrial, aquatic, and atmospheric), and monitoring (quantification and identification). A single report can feature in more than one study type; for example, studies may feature both uptake and fate or uptake and toxicity, as in the works of Rubin et al. (2023) or Heinze et al. (2021), respectively.215,231 For each publication, Table 3 reports the research question being addressed, the type of sample or matrix into which the MNPs were intentionally spiked or introduced, the use (as a tracer or for quality control), the polymer type and state (pristine, doped, or functionalized), the physicochemical properties characterized and the methods used for characterization. If the material was purchased, the manufacturer or company is indicated; otherwise, the top-down or bottom-up production method is indicated. As a minimum requirement, the studies selected for our review report the characterization of at least one MNP property. This is notable because a significant fraction of the literature purporting to use reference and test MNPs do not characterize any of their properties34 and were excluded from our analysis. For each of these selected studies, the properties of the reference MNPs characterized and the analytical techniques used to measure these properties are indicated. In addition, the key property/properties responsible for the outcomes or conclusions of the study are identified. Polymer type (i.e., chemical structure) and particle size are the most frequently characterized properties. In Table 3, size stands out as a key parameter in circa 70% of the studies. Shape (sphere, fragment, fiber) follows in relevance, with 19 mentions. Surface and bulk properties are reported at significantly lower frequencies. Similarly, the properties of the media where MNPs are stored or spiked are also frequently underreported, often being limited to the type of solvent/media and the MNP concentration (particle- or mass-based).Reference and test MNPs are indispensable for advancing research by enabling controlled experiments. They facilitate systematic investigations into the fate and effects of MNPs while ensuring experimental reproducibility. It is important to recognize that reference and test MNPs are used differently depending on the study type: in our classification, toxicity studies assess the impact of MNPs on humans219,220 and other living organisms210,211 using in vitro and in vivo models. In these experiments, reference MNPs and their detailed characterization are critical for controlling the concentration, type, and properties of particles used as tracers, allowing researchers to correlate observed toxicological outcomes with specific physicochemical properties. Proper characterization of reference MNPs is essential to avoid artifacts caused by contaminants, such as surfactants used in particle suspensions, or unintended surface modifications introduced during production. Uptake studies differ from toxicity studies in that they focus on the entry (ingestion, inhalation, cellular uptake) and distribution of MNPs within organisms, whereas fate studies focus on the distribution, transport, accumulation, and elimination of MNPs in a given environmental matrix (i.e. water or terrestrial environments, or atmosphere). In uptake and fate studies, MNPs are often used to track the translocation of the particles, so MNPs need to be detected or visualized in the matrix. Monitoring studies aim to identify and/or quantify MNPs in the environment and living organisms, so they typically employ MNPs as a quality control333 to validate or demonstrate the feasibility of sample preparation methods,240 extraction protocols,249,251 detection techniques,61,67 or quantification methods.253,334,335 In this context, MNP recoveries, assessed by adding and recovering known quantities of MNPs from test media, provide a metric for the under- or overestimation of MNPs. This allows the efficacy of the method to be gauged as a function of the MNP characteristics and allows results to be reported more quantitatively and with a higher degree of confidence. Similarly, reference MNPs can also be used as internal standards to correct for matrix effects and instrumental drifts.198,336 Moreover, reference MNPs are also needed to calibrate or to obtain reference spectra of MNPs with different characteristics. This information improves the accuracy of polymer identification/detection when considering possible effects of, for example, color, opacity, texture, crystallinity, presence of additives or contaminants, the matrix, etc.337 The use of well-characterized reference and test MNPs ultimately allows researchers to assess the extent to which different studies can be meaningfully compared, thereby promoting a better understanding of the relationships between MNP properties and their environmental or biological fate and impact.
9 Summary
In the past 5–10 years there has been an awareness that the quantity of MNPs entering the environment is increasing rapidly, fueling an explosion in research efforts directed towards understanding their behavior and effects. However, the inherent challenges of MNPs collection, identification and quantification in different environmental compartments has led most researchers to focus on analytical method development and lab-based studies where reference and test MNPs are either purchased from commercial vendors or produced in house. This review was motivated by the need not only to compare, contrast and assess the existing MNPs production methods, but also to evaluate the properties and applicability of the existing MNPs to answer diverse research questions.The initial part of this review overviews existing commercial MNPs (a detailed list is included in ESI† 1) and their limitations, as well as the means of producing “in house” reference and test MNPs. There are a wide variety of top-down methods that create MNPs by applying an external force to fragment larger sized plastics (Table 1); grinding and milling the most common methods, often under cryogenic conditions. Top-down methods are particularly well-suited to produce MNPs of random shapes and sizes that reflect those encountered in the environment. In contrast, bottom-up methods rely on the formation of MNPs from molecular building blocks (Table 2). These methods provide greater opportunities to control key MNP properties, particularly size, and can be adapted to include different labels that facilitate the detection and quantification of MNPs in complex environmental matrices. However, bottom-up methods are invariably restricted to the synthesis of spherical particles whose surface properties can sometimes be compromised, most notably by the adsorption of surfactants. Table 4 provides a concise summary of the characteristics, advantages and limitations of the two types of production methods.
| Parameter | Top-down | Bottom-up |
|---|---|---|
| Shape | Fragment | Sphere |
| MPs production rate | High | High |
| NPs production rate | Low | High |
| Surface properties | Irregular, oxidation possible | Texture can be controlled, surfactants may be present |
| Reproducibility | Poor | High |
| Labeling options | Only surface, post-synthesis | Bulk and surface |
| Advantages | • Instrumentation is often widely available | • Faster and scalable |
| • Generally simple procedure | • Controllable size | |
| • Applicable to most polymer/plastic types | • Labels can be incorporated | |
| • Higher environmental relevance | ||
| Limitations | • Sieving or filtration is required for size fractionation | • Shape is mostly limited to spheres |
| • Potential metal contamination | • Use of non-environmentally friendly polymer-specific solvents | |
| • Labelling is difficult | • Requires some experience in polymer chemistry | |
| • No control over additive content |
The wide range of properties that collectively describe MNPs can present a daunting challenge for most researchers, who do not have an unlimited array of characterization/detection techniques available. The second part of this review has surveyed the literature to ascertain the applications of reference and test MNPs to answer key research questions, divided into four study types (fate, toxicity, uptake and monitoring), identifying properties that are ubiquitously important to characterize regardless of the study type (e.g. size), as well as those that are mostly relevant to certain types of studies (e.g. bulk polymer composition for toxicity and monitoring). Table 5 provides an overview of the MNP properties that need to be characterized depending on the study type (toxicity, uptake, fate, and monitoring) and the most common techniques that are appropriate to determine them depending on the particle size.
| Study type | Property | Analytical techniques | ||||
|---|---|---|---|---|---|---|
| Toxicity | Uptake | Fate | Monitoring | NP | MP | |
| a Includes the characterization of additives, residual monomers and co-polymers. | ||||||
| XX | XX | XX | XX | Polymer type | Py- and TED-GC/MS | |
| Raman and FTIR | ||||||
| XX | XX | XX | XX | Size | DLS, NTA, spICP-MS | |
| Electron microscopy | Optical microscopy | |||||
| XX | XX | XX | XX | Shape | Electron microscopy | Optical microscopy |
| XX | XX | XX | — | Surface charge | Zeta potential measurement (ζ) | |
| X | X | X | — | Surface chemistry | XPS | |
| — | — | X | X | Density | Density gradient | |
| X | — | X | — | Crystallinity | DSC and Raman | |
| XX | — | — | X | Bulk compositiona | GC/MS, LC/MS | |
Table 3 showed that most studies with characterization data provide information about at least 2 or 3 properties of MNPs, with a significantly smaller number of studies characterizing 4 or more properties. Toxicological studies are the most characterization intensive, due to the wide range of MNP properties that can contribute to adverse effects in humans and environments. In conclusion, regarding in MNPs studies:
• Polymer type, size, and shape of reference and test MNPs should always be characterized. These properties are relevant in all types of studies, improve the comparability across studies and are relatively easy to measure using available techniques.
• Surface properties are important to determine in almost all study types, as they are responsible for particle stability and strongly influence MNP interactions with other species.
• Bulk plastic composition, especially identification and concentration of additives and residual monomers, should be assessed in monitoring studies and is critical to toxicity studies, but not for other study types.
• Bulk physical properties are generally not needed with some exceptions. For example, density should be considered in specific cases like fate and transport studies, or monitoring studies where the density influences the efficiency of MNPs extraction from the sample matrix. Crystallinity should be reported when the study involves the potential for MNP biodegradation and/or fragmentation.
• Particle concentration and dose as well as solution chemistry are not MNP properties, but these parameters are key for some study types. Thus, knowing MNP dose is crucial when spiking for detection method validation or for in vitro toxicity studies, and solution chemistry must be well-defined for toxicity studies.
10 Future directions and needs
This review concludes by discussing some of the future directions and open issues related to the production, characterization and use of reference and test MNPs, including several research opportunities, the need for more of both commercially available and in-house, and MNPs suggestions best practices for MNP studies, highlighting the need for quality assurance and quality control. The hope is that the implementation of these practices will help to improve the overall data quality in the field of MNP research as a route to providing a better understanding and an improved, more quantitative assessment of the risks (or not) posed by different MNPs.10.1 Commercial MNPs
Commercially available MNPs are dominated by PS and PE, usually spherical and pristine. There is therefore an obvious need to expand the range of commercially available MNPs to encompass a wider range of polymer types and shapes. Commercial MNPs should also include irregular fragments in the low microsized and nanosized ranges to mimic the shape and size range of environmental MNPs. Finally, disclosing the details of the polymer composition (e.g. the type and concentration of any chemical additives) and/or the composition of the matrix/suspension containing the MNPs as standard practice, would be extremely valuable for researchers.Besides these technical considerations, discussions between academic researchers and plastics manufacturers should be strengthened to foster the production of large quantities of MNP particles with controlled chemical and physical properties. Similarly, encouraging interdisciplinary interactions between environmental scientists and analytical chemists, polymer chemists or materials scientists would be invaluable, as the former have specific needs that could be addressed with the expertise of the latter. The involvement of institutions such as the Joint Research Center (JRC) of the European Commission, metrology institutes (e.g. NIST), standardization bodies and technical committees will be crucial to this endeavor. Once the proof-of-concept and suitability of a production method have been demonstrated, the aim should be to produce certified reference materials (CRMs) of varying characteristics following the validation and certification processes outlined in guides such as ISO 17034:201647, ISO Guide 35:201748, and JJF1343-201249. Although we acknowledge that certification is expensive, commercially available reference MNPs with a certificate of analysis of the essential properties highlighted in this review would be highly beneficial for interlaboratory comparisons, standardization, and validation of analytical procedures.
An underutilized benefit of well-controlled and well-characterized commercial MNPs (ideally certified) is their facilitation for inter and intra laboratory comparisons. There are already an enormous number of studies describing the behavior and effects of MNPs, but results obtained in one study are often hard to compare with other studies even if the research question and matrix are nominally similar. If a commercial MNP is included as part of a study, then this data will provide a benchmark to compare results from these studies with other studies where the same commercial MNP was employed.
One challenge with commercial MNPs is the lack of consensus as to the ability of these well-defined MNPs to mimic the behavior of MNPs produced by natural processes. One issue is the different shapes, with commercial MNPs being usually spherical due to their bottom-up production, in contrast to the irregular shapes of naturally-occurring MNPs. Thus, it would be valuable to determine if the behavior of spherical MNPs can be directly extrapolated to MNPs generated naturally or by top-down means with equivalent spherical diameter.
10.2 “Realistic” MNPs
“Realistic” MNPs can be obtained by extraction from the environment, an option largely underexplored at the moment due to the intensive labor involved and the low rate of recovery. The harsh chemicals sometimes used to perform extraction from complex matrices may also unintentionally alter MNP properties. For monitoring studies, this may make the detection of the MNPs with spectroscopic and spectrometric instruments challenging as the polymer fingerprint(s) may have changed. Using reference MNPs as an internal control, subjecting them to the same extraction process, and then characterizing the MNPs produced and comparing these results to the pristine reference MNPs is a useful test to ensure no measurable alterations occurred during the extraction process. One interesting alternative might be to collect MNPs through airborne samples.The production of commercial MNPs almost exclusively involves bottom-up methods. In contrast, more “realistic” MNPs representative of the heterogeneous shapes and polydispersed sizes found in the environment are currently produced predominantly in house using top-down approaches. One overarching challenge in the fabrication of realistic MNPs is the low-throughput of top-down methods which makes larger-scale, multi-laboratory and round robin type studies difficult. Top-down production typically has high yields for fragments of sizes above a few microns, but these yields drop dramatically for nanosized particles (≪1%).37 Therefore, developing high-yield methods to fabricate nanosized fragments is essential to produce kilogram quantities of MNPs in a reasonably rapid and cost-effective manner, suitable for widespread use. Herein, we note that in the most common top-down methods, (e.g. milling) the production yields (mass of MNP divided by the mass of starting material) and the production rates (amount of product per unit time) are rarely detailed, despite being essential to allow a particular method to be scaled-up. We suggest systematically reporting throughput values clearly, ideally as a function of the MNP size range(s) produced. One promising approach to increase the yield of MNPs would be to use artificial weathering as a means to embrittle bulk plastic before the top-down method is used to break the plastic down and create MNPs.102 Some relatively under-utilized top-down methods appear amenable to producing reasonable quantities of weathered MNPs directly, such as ultrasonication, where fragmentation and oxidation occur simultaneously.71,77 However, care should be taken to determine the extent to which the artificial weathering of the bulk plastic alters the properties of the MNPs.
In some specific cases, production hurdles have hampered the ability to create reference MNPs. One example is in the production of MNP fibers, which is being hindered by two major interconnected challenges. First, extrusion/spinning processes with a reasonable throughput must be developed or adapted to obtain polymer filaments with diameters ranging from nanometers to a few micrometers, representative of the MNP fiber diameters found in the environment, but significantly smaller than the 10 to 40 μm range produced by the textile industry. In this respect, electrospinning was identified as a potentially viable process to produce polymer filaments down to about 100 nm in diameter, something which is not achievable with traditional extrusion processes such as melt-spinning. The second, and perhaps most challenging aspect is to develop efficient methods to cut the filaments at small and regular lengths (<1 μm) capable of producing batches of very small MNP fibers. For almost all top-down methods, however, there is a need to harmonize the level of detailed reporting of experimental parameters so that greater control and reproducibility can be exerted over the characteristics of the MNPs produced, particularly particle size distributions.
A detailed discussion on the development of unique types of reference MNPs, such as tire wear particles made of rubber, was beyond the scope of this review. However, we would like to highlight that some options do exist to obtain these rubber particles, including direct collection on the road and top-down approaches such as, abrasion of tires in the laboratory or cryomilling a mixture of tires from different manufacturers.338,339 The ISO/TS 22638:2018 protocol describes a method for the standardized production of representative tire and road wear particles without any contamination issues that could arise from the use of a road simulator. However, the main challenge in the production of reference tires MNPs lies in the varying composition of tires caused by the different manufacturing processes.340
10.3 Storage stability of MNPs
The stability of both commercially available and in-house generated MNPs can change over time. This is because storage conditions may alter the properties of MNPs, resulting for example in their agglomeration. For this reason, it is necessary to assess the optimal storage conditions of MNPs to provide recommendations to the community. This same information can also be used as the basis to improve the colloidal stability of test and reference MNPs. Various approaches have been developed to improve MNP colloidal stability, including the use of organic solvents, surfactants or the surface oxidation of MNPs (e.g. with ozone). However, these strategies may compromise the environmental relevance of the particles, potentially skewing the outcome of the studies. There is therefore a need to effectively disperse MNPs while not compromising their environmental relevance. Moreover, the stability of reference and test MNPs, whether stored in a solution, as powder, or embedded in a solid matrix, is often overlooked for both commercially-available or in-house produced materials. It is essential that stability assessment becomes part of the standard characterization of MNPs and is reported consistently. To this end, the ISO 33405:2024 protocol provides some guidelines for the characterization of the stability and homogeneity of reference materials.10.4 MNP labelling
Multilabeled or multifunctional test and reference MNPs, benefitting from the advantages of different labels (i.e. for MNP imaging, quantification, sizing), would be useful to expand our understanding of transport, degradation, fragmentation and uptake mechanisms, particularly in complex matrices. Notably, the combination of labels that enable both quantitative assessment (i.e. metal or isotope tags) as well as visualization (i.e. fluorescent, colored) of MNPs offers the most interesting synergies. For example, Luo et al. (2022) synthesized metal-tagged fluorescent MNPs, which allowed the in situ visualization of the particles and their further quantification by ICP-MS.150 Furthermore, multifunctional MNPs could enable the comparison and performance validation of different analytical techniques. For example, MNPs with a color tag visible to optical microscopy/spectroscopy techniques and a stable isotope label identifiable by thermo-analytical techniques could be used as internal standard for both instruments, opening the door to method cross-validation and harmonization. For these reasons, the commercial availability of these more complex, multi-modal MNPs is needed.The labelling of macroscopic sized plastics would also help MNP research efforts. This is a consequence of the low production rates of MNPs from macroscopic plastics due to natural chemical or physical degradation processes in the environment.180 Moreover, quantification of these MNP production rates tends to be obscured by the complex, heterogeneous media into which they are released. Accurate, quantitative information on MNP release rates in realistic environments and the factors that influence these release rates is therefore difficult to obtain. This knowledge gap could be addressed by designing metal-doped or stable isotope labeled macroscopic plastics that facilitate the detection and quantification of MNPs released as plastics break down in different release scenarios (e.g. sand abrasion, natural sunlight). Information gained from these types of studies would also be valuable for developing emission inventories for various sectors and compartments, which are essential inputs for environmental models that inform policy. For instance, sources such as tire abrasion, agricultural dust, and ocean spray have been identified as contributors to atmospheric MNPs. However, current estimates of emission rates from these sources carry up to 100% uncertainty.341
10.5 MNP weathering
In the environment MNPs often transform from the initial state in which they enter the environment because of natural weathering, but protocols for weathering MNPs are often lacking and incompletely described and therefore difficult to replicate in the absence of more widely accepted, standardized procedures. In part, this reflects the wide variety of ways in which MNPs can be naturally weathered in the environment, either by chemical (e.g. oxidation), physical (e.g. abrasion) or biological (e.g. microbial activity) processes. Moreover, even for a specific type of weathering experimental parameters may vary (e.g. UV light sources of different spectral range or power for photochemical weathering). Given this variability, the properties of particles that have nominally been weathered under the same conditions will inevitably differ amongst studies, hampering meaningful cross-comparisons. So, while we encourage the accurate reporting of the detailed procedures used to effect weathering, reporting the properties of the weathered MNPs as compared to the pristine MNPs before weathering is the critical information needed to compare results across different studies and to understand the relationships between accelerated and natural weathering. For example, it can help determine the timescale relationship between MNPs artificially weathered in a lab and those weathered by natural sunlight to achieve similar changes in physicochemical properties. Indeed, the importance of reporting particle characterization and experimental protocols for MNP weathering has been highlighted in a recent review as the key knowledge gaps in this area.17110.6 Establishing property–function relationships for MNP studies
The enormous range of polymer types places a significant burden on researchers. In this vein, if property–function relationships could be established that are independent, or at least largely independent of polymer type, then this would reduce the number of MNP studies needed. For example, if similarly sized NP spheres of different polymer types (e.g. PVC, PE, PET) could be differentially weathered in such a way that they all possessed the same surface charge and were then found to exhibit the same behavior in situations where size, shape and surface chemistry are determinant properties (e.g. transport), this would allow results from one MNP study to be meaningfully extrapolated to other polymer types. In a similar vein, there is a reasonable expectation that as MNPs are increasingly weathered in the environment, polymer type will become less important in determining behavior. Unfortunately, to-date most MNP studies have been conducted for one polymer type with a unique set of MNP characteristics as opposed to either multiple polymer types with similar characteristics or a MNPs of the same polymer type each with different characteristics (e.g. differently weathered MNPs of the same polymer type). This absence of internal diversity of MNP polymer types and characteristics has hindered the development of cross-cutting MNP relationships of this type.Another strategy that could be employed to reduce the experimental burden would be to categorize MNPs based on both their properties (e.g. size, shape) and reactivity (e.g. ROS production) and then to only study the most representative MNP. This approach would enable “grouping” and read-across strategies, similar to those adopted in other fields, such as nanotoxicity.342,343
10.7 MNP database
One important step towards the standardization of reference materials for MNPs would be the creation of a FAIR (findable, accessible, interoperable, reusable) and open database or data repository for the storage and reuse of relevant information regarding reference and test MNPs. Indeed, this was a specific recommendation of the American Chemistry Council in 2022 tasked with establishing guidelines for reference MNPs.36 This information should encompass, at a minimum, the production method or the manufacturer (and batch), the physicochemical properties that have been characterized and by what method(s), the outcome of studies performed, as well as the experimental protocols, including details of the matrix where the MNP study was conducted (i.e. pH, conductivity, etc.).344,345 The establishment of such a database would significantly facilitate the comparison of reference and test MNPs produced by different means. This information in turn could open up the opportunity for meta-analysis and modelling studies aiming at data gap identification and predictions, insights that would be particularly useful in disentangling the multiplicity of MNP variables that are involved in toxicity studies.346 A number of analogous databases have been developed for nanomaterials, and it may be feasible to reuse similar structures for reference MNPs.345,34610.8 MNP toxicity studies
Results from MNP toxicity studies are often contradictory and inconsistent, even when the studies are nominally conducted on the same organism or cell line, using the same nominal MNPs at similar concentrations and exposures times, etc. One of the difficulties arises from the variable stability, homogeneity and composition of the MNP suspensions used in toxicity studies. The use of surfactants to produce stable MNP suspensions is strongly discouraged. If surfactants were necessary for MNP production (as in some bottom-up methods) or dispersion, they must be removed prior to MNP use, as they modify surface properties (e.g. surface charge) and, thus, significantly distort the characteristics (e.g. stability) and behavior (e.g. uptake) of the MNPs. In situations where surfactants are needed to create stable MNP suspensions toxicity studies have no environmental relevance because the MNPs would not be exposed to the organism in the environment and therefore pose no risk, at least as a colloidal particle (risk = hazard × exposure). We hypothesize that another reason for the discrepancy in reported toxicity of MNPs is the variability in plastic composition. This includes the presence of chemical additives, which can leach from MNPs, and play a determinant role in regulating the measured MNP toxicity. For example, plasticizers are a significant component in many plastic materials (e.g., typically about 30–35 wt% for PVC) and many plasticizers, such as di(2-ethylhexyl) phthalate and some of its metabolites, are endocrine disruptors. Top-down methods of generating MNPs use commercial plastics often of unknown composition, while bottom-up approaches offer much better control over the composition of MNPs. Creating identical MNPs but with differing concentrations of additives will help to discern if the physical or the chemical properties of the particles control their toxicity. As an extension of this idea, the creation of MNPs with a single additive or with multiple additives would allow the toxicity of additives to be studied both in isolation, while also serving as a platform to identify synergistic or antagonistic effects when they are present in more complex, but industrially relevant mixtures. In addition, the effect of possible microbial/endotoxin contamination must also be evaluated, as it may be a cause of divergent results.10.9 MNPs as internal standards and positive controls for QA/QC
Well-characterized MNPs, either commercial or lab synthesized, should be used as internal standards or positive controls in studies designed to estimate the degree of analytical uncertainty (accuracy), as well as identify and quantify instrumental drift and thereby improve data quality, particularly when multi-step sample processing is needed as a prerequisite to analysis. While the QA/QC measures applied in MNP research mainly consist of analyzing different types of blanks and preventing contamination during the sampling collection and in the lab,26 an evaluation of MNP losses during both sample preparation and detection is not systematically carried out. In situations where spectroscopic methods are used for MNP detection, a known number of easily identifiable and clearly distinguishable MNPs (e.g. colored spheres) could be added as MNP standards to the sample before processing.51 Recognizing that some or all of characteristics associated with the MNPs being quantified will not be known, the operator should still be able to make an informed guess to ensure that the MNP standards are as similar as possible to the environmental MNPs expected in the samples in terms of polymer type, size, etc. By determining the recovery of the MNP standards at the end of the sample preparation this approach offers the best means to estimate MNP extraction efficiency. This information would then indicate whether the native MNPs in samples have been quantitatively extracted and thereby provide a means to estimate sample-specific recoveries, helping to identify poorly prepared samples. Although this information is a useful gauge of the MNP loss rate during the whole analytical process, these values should not be used to correct results in an absolute quantitative fashion, as the analytes and the standards are not strictly identical.10.10 Standardization of MNP characterization/detection
A significant gap in the characterization and detection of MNPs is the lack of harmonized and standardized operating procedures, which are crucial for ensuring quality control and comparability of results, minimizing errors and misunderstandings. Many initiatives are ongoing, promoted by different research projects and technical committees of standardization bodies, but only a few standards exist, and these are all relatively new. The existing standards have been compiled into a table available in an online repository (see Data availability) ISO has two documents, 24187:2023 and 5667-27, about principles for the analysis of microplastics present in the environment and water, respectively, one with a method to determine mass concentration of tire and road wear particles, 21396:2017, and two complementary drafts, 16094-2 and -3,347,348 for the analysis of microplastics in water, covering sampling, vibrational spectroscopy analysis, and thermos-analytical methods, respectively. ASTM has already three complementary standards: D8332-20 for the collection of aqueous samples with suspended solids for identification and quantification of microplastic particles and fibers, D8333-20 for preparation of these type of samples for analysis by microspectroscopy or Py-GC/MS, and D8489-23e1, a complementary test method for determination of size, distribution, shape, and concentration in waters using a dynamic image particle analyzer. ASTM also suggests how to prepare reference samples to calibrate and assess the efficiency of these collection, preparation, and identification methods described in D8402-23. These efforts will hopefully provide more guidance for MNP characterization/detection soon. Crucially, recommendations arising from these efforts should equally by both the scientific and industrial communities, fostered by regulation.Abbreviation
| ABS | Acrylonitrile butadiene styrene |
| AFM | Atomic Force Microscopy |
| AF4 | Asymmetric Flow Field Flow Fractionation |
| APS | Aerodynamic Particle Size Spectrometry |
| ASTM | American Society for Testing and Materials |
| ATR | Attenuated Total Reflectance |
| BAM | Federal Institute for Materials Research and Testing (German: Bundesanstalt für Materialforschung und -prüfung) |
| BET | Brunauer–Emmett–Teller |
| CCC | Critical coagulation concentration |
| CRM | Certified reference materials |
| CSC | Critical stabilization concentration |
| DLS | Dynamic light scattering |
| DOTA | Dodecane tetraacetic acid or tetraxetan |
| DSC | Differential Scanning Calorimetry |
| EDX | Energy-Dispersive X-Ray Spectroscopy |
| ELS | Electrophoretic Light Scattering |
| EM | Electron Microscopy |
| FE-SEM | Field Emission-Scanning Electron Microscopy |
| FFF | Field-Flow Fractionation |
| FluidFM | Fluidic force microscopy |
| FPA | Focal plane array |
| FTIR | Fourier Transform Infrared Spectroscopy |
| GPC | Gel Permeation Chromatography |
| HAADF | High-angle annular dark-field imaging |
| HPLC | High-performance Liquid Chromatography |
| ICP-MS | Inductively Coupled Plasma-Mass Spectrometry |
| JRC | Joint Research Center |
| LC-UV-MS/MS | Liquid Chromatography-Ultraviolet-Mass Spectrometry/Mass Spectrometry |
| LD | Laser Diffraction |
| LDIR | Laser Direct Infrared Spectroscopy |
| LEXRF | Low-Energy X-Ray Fluorescence |
| MADLS | Multi Angle Dynamic Light Scattering |
| MIR | Mid Infrared |
| MP | Microplastic particle |
| MNP | Micro- and nanoplastic particle |
| NB | Nile Blue |
| NIST | National Institute of Standards and Technology |
| NMR | Nuclear Magnetic Resonance |
| NP | Nanoplastic particle |
| NR | Nile red |
| NTA | Nanoparticle tracking analysis |
| PA | Polyamide |
| PA6 | Nylon 6 or polyamide 6 or polycaprolactam |
| PA6,6 | Nylon 6,6 or polyamide 6,6 |
| PAN | Polyacrylonitrile |
| PBAT | Polybutylene adipate terephthalate |
| PBS | Phosphate-buffered saline |
| PC | Polycarbonate |
| PDI | Polydispersity index |
| (HD-/LD-)PE | (High density-/low density) polyethylene |
| PES | Polyethersulfone |
| PET | Polyethylene terephthalate |
| PHBV | Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) |
| PLA | Polylactic acid |
| PMMA | Polymethyl methacrylate |
| PNC | Particle number concentration |
| PP | Polypropylene |
| PS | Polystyrene |
| PSD | Particle size distribution |
| PTFE | Polytetrafluoroethylene (Teflon) |
| PU | Polyurethane |
| PVC | Polyvinyl chloride |
| PVDC | Polyvinylidene chloride |
| PVP | Polyvinylpyrrolidone |
| Py-GC/MS | Pyrolysis-Gas Chromatography-Mass Spectrometry |
| P2VP | Poly(2-vinylpyridine) |
| QA/QC | Quality assurance/quality control |
| ROS | Reactive oxygen species |
| SAXS | Small-Angle X-Ray Scattering |
| SDS | Sodium dodecyl sulphate |
| SEC | Size Exclusion Chromatography |
| SEM | Scanning Electron Microscopy |
| SERS | Surface-Enhanced Raman Spectroscopy |
| SLS | Static Light Scattering |
| SMPS | Scanning Mobility Particle Sizer |
| spICP-MS | Single Particle Inductively Coupled Plasma Mass Spectrometry |
| SRM | Standard reference material |
| SRS | Stimulated Raman scattering |
| STEM | Scanning Transmission Electron Microscopy |
| STXM | Scanning Transmission X-Ray Microscopy |
| TED-GC/MS | Thermal Extraction Desorption-Gas Chromatography/Mass Spectrometry |
| TEM | Transmission Electron Microscopy |
| TGA | Thermogravimetric Analysis |
| THF | Tetrahydrofuran |
| TPEF | Two-Photon Excitation Microscopy (fluorescence) |
| UCNP | Upcon® upconverting nanoparticle |
| UPLC-MS/MS | Ultra-High Performance Liquid-Chromatography-Mass Spectrometry/Mass-Spectrometry |
| UV | Ultraviolet Light |
| UV-vis | Ultraviolet-Visible Spectroscopy |
| WWTP | Wastewater treatment plant |
| XPS | X-Ray Photoelectron Spectroscopy |
| XRD | X-Ray Diffraction |
| ζ | Zeta-potential |
Data availability
The sources of the data retrieved in ESI† 1 are listed in the document with a URL link. The list of the commercially-available MNPs (Supplementary Material 1) can also be accessed using the DOI: https://doi.org/10.5281/zenodo.14969091 and referred to as: Crosset-Perrotin G, Moraz A, Portela R, Alcolea-Rodriguez V, Burrueco-Subirà D, Smith C, et al. Commercially available microplastics and nanoplastics (MNPs) for use as test or as reference materials. Zenodo; 2025. The list of standards related to microplastics can be accessed using the DOI: https://doi.org/10.5281/zenodo.15086119 and referred to as: Portela R. List of standards related to microplastics. Zenodo; 2025.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by US National Science Foundation (Grant No. 2114682) as part of INFRAMES (AccelNet Implementation: International Network For Researching, Advancing and Assessing Materials for Environmental Sustainability). DHF acknowledges support from the US National Science Foundation (Grant No. 2003481). HF acknowledges support from the US National Science Foundation (Grant No. 2145532). GCP and AM acknowledge partial support from the Swiss Federal Office for the Environment (Grant No. 20.0093.PJ/C3DDE1CBA and 20.0093.PJ/BAFU-D-89643401/443, respectively). VAR and RP were supported by the EU H2020 Project PlasticsFate (GA 95921).References
- Plastics – the Facts 2022, PlasticsEurope, 2022 Search PubMed.
- A. L. Andrady and M. A. Neal, Applications and societal benefits of plastics, Philos. Trans. R. Soc., B, 2009, 364, 1977–1984 CrossRef CAS PubMed.
- M. Elsabahy and K. L. Wooley, Design of polymeric nanoparticles for biomedical delivery applications, Chem. Soc. Rev., 2012, 41, 2545–2561 RSC.
- B. M. Jarai, E. L. Kolewe, Z. S. Stillman, N. Raman and C. A. Fromen, in Nanoparticles for Biomedical Applications, Elsevier, 2020, pp. 303–324 Search PubMed.
- L. M. Hernandez, N. Yousefi and N. Tufenkji, Are There Nanoplastics in Your Personal Care Products?, Environ. Sci. Technol. Lett., 2017, 4, 280–285 CrossRef CAS.
- M. Faber, M. Marinković, E. de Valk and S. Waaijers-van der Loop, Paints and microplastics. Exploring the possibilities to reduce the use and release of microplastics from paints. Feedback from the paint sector, Rijksinstituut voor Volksgezondheid en Milieu, 2021 Search PubMed.
- N. B. Hartmann, T. Hüffer, R. C. Thompson, M. Hassellöv, A. Verschoor, A. E. Daugaard, S. Rist, T. Karlsson, N. Brennholt, M. Cole, M. P. Herrling, M. C. Hess, N. P. Ivleva, A. L. Lusher and M. Wagner, Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris, Environ. Sci. Technol., 2019, 53, 1039–1047 CrossRef CAS PubMed.
- ISO/TR 21960, https://www.iso.org/standard/72300.html, (accessed 6 February 2025).
- M. MacLeod, H. P. H. Arp, M. B. Tekman and A. Jahnke, The global threat from plastic pollution, Science, 2021, 373, 61–65 CrossRef CAS PubMed.
- J. R. Jambeck, R. Geyer, C. Wilcox, T. R. Siegler, M. Perryman, A. Andrady, R. Narayan and K. L. Law, Plastic waste inputs from land into the ocean, Science, 2015, 347, 768–771 CrossRef CAS PubMed.
- C. M. Rochman and T. Hoellein, The global odyssey of plastic pollution, Science, 2020, 368, 1184–1185 CrossRef CAS PubMed.
- C. M. Rochman, Microplastics research—from sink to source, Science, 2018, 360, 28–29 CrossRef CAS PubMed.
- A. L. Andrady, Microplastics in the marine environment, Mar. Pollut. Bull., 2011, 62, 1596–1605 CrossRef CAS PubMed.
- B. Gewert, M. M. Plassmann and M. MacLeod, Pathways for degradation of plastic polymers floating in the marine environment, Environ. Sci.: Processes Impacts, 2015, 17, 1513–1521 RSC.
- A. A. Koelmans, P. E. Redondo-Hasselerharm, N. H. M. Nor, V. N. de Ruijter, S. M. Mintenig and M. Kooi, Risk assessment of microplastic particles, Nat. Rev. Mater., 2022, 7, 138–152 CrossRef.
- A. L. Andrady, The plastic in microplastics: A review, Mar. Pollut. Bull., 2017, 119, 12–22 CrossRef CAS PubMed.
- S. M. Abel, S. Primpke, F. Wu, A. Brandt and G. Gerdts, Human footprints at hadal depths: Interlayer and intralayer comparison of sediment cores from the Kuril Kamchatka trench, Sci. Total Environ., 2022, 156035 CrossRef CAS PubMed.
- D. Materić, E. Ludewig, D. Brunner, T. Röckmann and R. Holzinger, Nanoplastics transport to the remote, high-altitude Alps, Environ. Pollut., 2021, 288, 117697 CrossRef PubMed.
- M. Bergmann, F. Collard, J. Fabres, G. W. Gabrielsen, J. F. Provencher, C. M. Rochman, E. van Sebille and M. B. Tekman, Plastic pollution in the Arctic, Nat. Rev. Earth Environ., 2022, 3, 323–337 CrossRef CAS.
- L. Li, Y. Luo, R. Li, Q. Zhou, W. J. G. M. Peijnenburg, N. Yin, J. Yang, C. Tu and Y. Zhang, Effective uptake of submicrometre plastics by crop plants via a crack-entry mode, Nat. Sustain., 2020, 3, 929–937 Search PubMed.
- N. Zolotova, A. Kosyreva, D. Dzhalilova, N. Fokichev and O. Makarova, Harmful effects of the microplastic pollution on animal health: a literature review, PeerJ, 2022, 10, e13503 CrossRef PubMed.
- H. A. Leslie, M. J. M. van Velzen, S. H. Brandsma, A. D. Vethaak, J. J. Garcia-Vallejo and M. H. Lamoree, Discovery and quantification of plastic particle pollution in human blood, Environ. Int., 2022, 163, 107199 CrossRef CAS PubMed.
- L. C. Jenner, J. M. Rotchell, R. T. Bennett, M. Cowen, V. Tentzeris and L. R. Sadofsky, Detection of microplastics in human lung tissue using μFTIR spectroscopy, Sci. Total Environ., 2022, 831, 154907 CrossRef CAS PubMed.
- A. Ragusa, A. Svelato, C. Santacroce, P. Catalano, V. Notarstefano, O. Carnevali, F. Papa, M. C. A. Rongioletti, F. Baiocco, S. Draghi, E. D'Amore, D. Rinaldo, M. Matta and E. Giorgini, Plasticenta: First evidence of microplastics in human placenta, Environ. Int., 2021, 146, 106274 CrossRef CAS PubMed.
- I. Dimante-Deimantovica, N. Suhareva, M. Barone, I. Putna-Nimane and J. Aigars, Hide-and-seek: Threshold values and contribution towards better understanding of recovery rate in microplastic research, MethodsX, 2022, 9, 101603 CrossRef PubMed.
- A. A. Koelmans, N. H. Mohamed Nor, E. Hermsen, M. Kooi, S. M. Mintenig and J. De France, Microplastics in freshwaters and drinking water: Critical review and assessment of data quality, Water Res., 2019, 155, 410–422 CrossRef CAS PubMed.
- S. Primpke, M. Fischer, C. Lorenz, G. Gerdts and B. M. Scholz-Böttcher, Comparison of pyrolysis gas chromatography/mass spectrometry and hyperspectral FTIR imaging spectroscopy for the analysis of microplastics, Anal. Bioanal. Chem., 2020, 412, 8283–8298 CrossRef CAS PubMed.
- S. Wieland, A. Balmes, J. Bender, J. Kitzinger, F. Meyer, A. F. Ramsperger, F. Roeder, C. Tengelmann, B. H. Wimmer, C. Laforsch and H. Kress, From properties to toxicity: Comparing microplastics to other airborne microparticles, J. Hazard. Mater., 2022, 428, 128151 CrossRef CAS PubMed.
- World Health Organization, Dietary and inhalation exposure to nano- and microplastic particles and potential implications for human health, World Health Organization, Geneva, 2022 Search PubMed.
- S. Atis, B. Tutluoglu, E. Levent, C. Ozturk, A. Tunaci, K. Sahin, A. Saral, I. Oktay, A. Kanik and B. Nemery, The respiratory effects of occupational polypropylene flock exposure, Eur. Respir. J., 2005, 25, 110–117 CrossRef CAS PubMed.
- H. Wiesinger, Z. Wang and S. Hellweg, Deep Dive into Plastic Monomers, Additives, and Processing Aids, Environ. Sci. Technol., 2021, 55, 9339–9351 CrossRef CAS PubMed.
- F. Yu, C. Yang, Z. Zhu, X. Bai and J. Ma, Adsorption behavior of organic pollutants and metals on micro/nanoplastics in the aquatic environment, Sci. Total Environ., 2019, 694, 133643 CrossRef CAS PubMed.
- M. Ogonowski, M. Wagner, B. Rogell, M. Haave and A. Lusher, Microplastics could be marginally more hazardous than natural suspended solids – A meta-analysis, Ecotoxicol. Environ. Saf., 2023, 264, 115406 CrossRef CAS PubMed.
- U. Rozman and G. Kalčíková, Seeking for a perfect (non-spherical) microplastic particle – The most comprehensive review on microplastic laboratory research, J. Hazard. Mater., 2022, 424, 127529 CrossRef CAS PubMed.
- T. Gouin, R. Ellis-Hutchings, L. M. Thornton Hampton, C. L. Lemieux and S. L. Wright, Screening and prioritization of nano- and microplastic particle toxicity studies for evaluating human health risks – development and application of a toxicity study assessment tool, Microplast. Nanoplast., 2022, 2, 2 CrossRef CAS PubMed.
- Microplastic Reference Materials - Invited Expert Workshop, American Chemistry Council, Atlanta, GA, USA, 2022 Search PubMed.
- L. Sørensen, M. H. Gerace and A. M. Booth, Small micro- and nanoplastic test and reference materials for research: Current status and future needs, Camb. Prism. Plast., 2024, 2, e13 CrossRef.
- S. Reynaud, A. Aynard, B. Grassl and J. Gigault, Nanoplastics: From model materials to colloidal fate, Curr. Opin. Colloid Interface Sci., 2022, 57, 101528 CrossRef CAS.
- C. R. de Bruin, E. de Rijke, A. P. van Wezel and A. Astefanei, Methodologies to characterize, identify and quantify nano- and sub-micron sized plastics in relevant media for human exposure: a critical review, Environ. Sci.: Adv., 2022, 1, 238–258 Search PubMed.
- J. Caldwell, P. Taladriz-Blanco, R. Lehner, A. Lubskyy, R. D. Ortuso, B. Rothen-Rutishauser and A. Petri-Fink, The micro-, submicron-, and nanoplastic hunt: A review of detection methods for plastic particles, Chemosphere, 2022, 293, 133514 CrossRef CAS PubMed.
- N. P. Ivleva, Chemical Analysis of Microplastics and Nanoplastics: Challenges, Advanced Methods, and Perspectives, Chem. Rev., 2021, 121, 11886–11936 CrossRef CAS PubMed.
- D. S. Moura, C. J. Pestana, C. F. Moffat, J. Hui, J. T. S. Irvine and L. A. Lawton, Characterisation of microplastics is key for reliable data interpretation, Chemosphere, 2023, 331, 138691 CrossRef CAS PubMed.
- V. N. de Ruijter, M. Hof, P. Kotorou, J. van Leeuwen, M. J. van den Heuvel-Greve, I. Roessink and A. A. Koelmans, Microplastic Effect Tests Should Use a Standard Heterogeneous Mixture: Multifarious Impacts among 16 Benthic Invertebrate Species Detected under Ecologically Relevant Test Conditions, Environ. Sci. Technol., 2023, 57, 19430–19441 CrossRef CAS PubMed.
- U. Rozman, T. Turk, T. Skalar, M. Zupančič, N. Čelan Korošin, M. Marinšek, J. Olivero-Verbel and G. Kalčíková, An extensive characterization of various environmentally relevant microplastics – Material properties, leaching and ecotoxicity testing, Sci. Total Environ., 2021, 773, 145576 CrossRef CAS PubMed.
- S. Wieland, A. F. R. M. Ramsperger, W. Gross, M. Lehmann, T. Witzmann, A. Caspari, M. Obst, S. Gekle, G. K. Auernhammer, A. Fery, C. Laforsch and H. Kress, Nominally identical microplastic models differ greatly in their particle-cell interactions, Nat. Commun., 2024, 15, 922 CrossRef CAS PubMed.
- A. E. Rubin, A. K. Sarkar and I. Zucker, Questioning the suitability of available microplastics models for risk assessment – A critical review, Sci. Total Environ., 2021, 788, 147670 Search PubMed.
- K. Waldschläger, M. Born, W. Cowger, A. Gray and H. Schüttrumpf, Settling and rising velocities of environmentally weathered micro- and macroplastic particles, Environ. Res., 2020, 191, 110192 CrossRef PubMed.
- J. N. Möller, M. G. J. Löder and C. Laforsch, Finding Microplastics in Soils: A Review of Analytical Methods, Environ. Sci. Technol., 2020, 54, 2078–2090 CrossRef PubMed.
- E. M. Crichton, M. Noël, E. A. Gies and P. S. Ross, A novel, density-independent and FTIR-compatible approach for the rapid extraction of microplastics from aquatic sediments, Anal. Methods, 2017, 9, 1419–1428 RSC.
- T. Mani, S. Frehland, A. Kalberer and P. Burkhardt-Holm, Using castor oil to separate microplastics from four different environmental matrices, Anal. Methods, 2019, 11, 1788–1794 RSC.
- M. Philipp, T. D. Bucheli and R. Kaegi, The use of surrogate standards as a QA/QC tool for routine analysis of microplastics in sewage sludge, Sci. Total Environ., 2022, 835, 155485 CrossRef CAS PubMed.
- M. G. J. Löder, H. K. Imhof, M. Ladehoff, L. A. Löschel, C. Lorenz, S. Mintenig, S. Piehl, S. Primpke, I. Schrank, C. Laforsch and G. Gerdts, Enzymatic Purification of Microplastics in Environmental Samples, Environ. Sci. Technol., 2017, 51, 14283–14292 CrossRef PubMed.
- S. Felsing, C. Kochleus, S. Buchinger, N. Brennholt, F. Stock and G. Reifferscheid, A new approach in separating microplastics from environmental samples based on their electrostatic behavior, Environ. Pollut., 2018, 234, 20–28 CrossRef CAS PubMed.
- J. Grbic, B. Nguyen, E. Guo, J. B. You, D. Sinton and C. M. Rochman, Magnetic Extraction of Microplastics from Environmental Samples, Environ. Sci. Technol. Lett., 2019, 6, 68–72 CrossRef CAS.
- A. L. Lusher, K. Munno, L. Hermabessiere and S. Carr, Isolation and Extraction of Microplastics from Environmental Samples: An Evaluation of Practical Approaches and Recommendations for Further Harmonization, Appl. Spectrosc., 2020, 74, 1049–1065 CrossRef CAS PubMed.
- L. Yu, B. Lei, S. Li, T. H. Nguyen, A. Jabersanri, F. Rezanezhad and P. Van Cappellen, Microplastic extraction from water, sediment, soil, and atmospheric deposition samples, DOI:10.5281/zenodo.10798963.
- H. E. Hadri, J. Gigault, B. Maxit, B. Grassl and S. Reynaud, Nanoplastic from mechanically degraded primary and secondary microplastics for environmental assessments, NanoImpact, 2020, 17, 100206 CrossRef.
- A. F. Astner, D. G. Hayes, H. O'Neill, B. R. Evans, S. V. Pingali, V. S. Urban and T. M. Young, Mechanical formation of micro-and nano-plastic materials for environmental studies in agricultural ecosystems, Sci. Total Environ., 2019, 685, 1097–1106 CrossRef CAS PubMed.
- A. Tamargo, N. Molinero, J. J. Reinosa, V. Alcolea-Rodriguez, R. Portela, M. A. Bañares, J. F. Fernández and M. V. Moreno-Arribas, PET microplastics affect human gut microbiota communities during simulated gastrointestinal digestion, first evidence of plausible polymer biodegradation during human digestion, Sci. Rep., 2022, 12, 528 Search PubMed.
- C. Jiménez-Arroyo, A. Tamargo, N. Molinero, J. J. Reinosa, V. Alcolea-Rodriguez, R. Portela, M. A. Bañares, J. F. Fernández and M. V. Moreno-Arribas, Simulated gastrointestinal digestion of polylactic acid (PLA) biodegradable microplastics and their interaction with the gut microbiota, Sci. Total Environ., 2023, 902, 166003 CrossRef PubMed.
- C. Park, D. Lim, S. M. Kong, N.-I. Won, Y. H. Na and D. Shin, Dark background–surface enhanced Raman spectroscopic detection of nanoplastics: Thermofluidic strategy, Water Res., 2023, 244, 120459 CrossRef CAS PubMed.
- S. Lievens, E. Vervoort, G. Poma, A. Covaci and M. V. D. Borght, A Production and Fractionation Protocol for Polyvinyl Chloride Microplastics, Methods Protoc., 2023, 6, 15 CrossRef CAS PubMed.
- T. Gardon, I. Paul-Pont, G. L. Moullac, C. Soyez, F. Lagarde and A. Huvet, Cryogrinding and sieving techniques as challenges towards producing controlled size range microplastics for relevant ecotoxicological tests, Environ. Pollut., 2022, 315, 120383 CrossRef CAS PubMed.
- L. A. Parker, E. M. Höppener, E. F. van Amelrooij, S. Henke, I. M. Kooter, K. Grigoriadi, M. G. A. Nooijens, A. M. Brunner and A. Boersma, Protocol for the production of micro- and nanoplastic test materials, Microplast. Nanoplast., 2023, 3, 10 CrossRef.
- L. Eitzen, S. Paul, U. Braun, K. Altmann, M. Jekel and A. S. Ruhl, The challenge in preparing particle suspensions for aquatic microplastic research, Environ. Res., 2019, 168, 490–495 CrossRef CAS PubMed.
- C. J. McColley, J. A. Nason, B. J. Harper and S. L. Harper, An assessment of methods used for the generation and characterization of cryomilled polystyrene micro- and nanoplastic particles, Microplast. Nanoplast., 2023, 3, 20 CrossRef CAS.
- M. S. M. Al-Azzawi, M. Kunaschk, K. Mraz, K. P. Freier, O. Knoop and J. E. Drewes, Digest, stain and bleach: Three steps to achieving rapid microplastic fluorescence analysis in wastewater samples, Sci. Total Environ., 2023, 863, 160947 Search PubMed.
- M. Piccardo, F. Provenza, E. Grazioli, A. Cavallo, A. Terlizzi and M. Renzi, PET microplastics toxicity on marine key species is influenced by pH, particle size and food variations, Sci. Total Environ., 2020, 715, 136947 CrossRef CAS PubMed.
- F. Blancho, M. Davranche, F. Fumagalli, G. Ceccone and J. Gigault, A reliable procedure to obtain environmentally relevant nanoplastic proxies, Environ. Sci.: Nano, 2021, 8, 3211–3219 RSC.
- A. K. Sarkar, A. E. Rubin and I. Zucker, Engineered Polystyrene-Based Microplastics of High Environmental Relevance, Environ. Sci. Technol., 2021, 55, 10491–10501 CrossRef CAS PubMed.
- E. Von Der Esch, M. Lanzinger, A. J. Kohles, C. Schwaferts, J. Weisser, T. Hofmann, K. Glas, M. Elsner and N. P. Ivleva, Simple Generation of Suspensible Secondary Microplastic Reference Particles via Ultrasound Treatment, Front. Chem., 2020, 8, 1–15 CrossRef PubMed.
- A. Dehaut, C. Himber, M. Colin and G. Duflos, Think positive: Proposal of a simple method to create reference materials in the frame of microplastics research, MethodsX, 2023, 10, 102030 CrossRef CAS PubMed.
- C. M. Knauss, C. F. Dungan and S. A. Lehmann, A Paraffin Microtomy Method for Improved and Efficient Production of Standardized Plastic Microfibers, Environ. Toxicol. Chem., 2022, 41, 944–953 CrossRef CAS PubMed.
- S.-Y. Lee, J. An and J.-H. Kwon, Sequential quantification of number and mass of microplastics in municipal wastewater using Fourier-transform infrared spectroscopy and pyrolysis gas chromatography-mass spectrometry, Environ. Pollut., 2023, 336, 122452 CrossRef CAS PubMed.
- M. Cole, A novel method for preparing microplastic fibers, Sci. Rep., 2016, 6, 1–7 CrossRef PubMed.
- R. Mossotti, G. D. Fontana, A. Anceschi, E. Gasparin and T. Battistini, Preparation and analysis of standards containing microfilaments/microplastic with fibre shape, Chemosphere, 2021, 270, 129410 CrossRef CAS PubMed.
- V. Tolardo, D. Magrì, F. Fumagalli, D. Cassano, A. Athanassiou, D. Fragouli and S. Gioria, In Vitro High-Throughput Toxicological Assessment of Nanoplastics, Nanomaterials, 2022, 12, 1947 CrossRef CAS PubMed.
- D. Magrì, P. Sánchez-Moreno, G. Caputo, F. Gatto, M. Veronesi, G. Bardi, T. Catelani, D. Guarnieri, A. Athanassiou, P. P. Pompa and D. Fragouli, Laser Ablation as a Versatile Tool To Mimic Polyethylene Terephthalate Nanoplastic Pollutants: Characterization and Toxicology Assessment, ACS Nano, 2018, 12, 7690–7700 CrossRef PubMed.
- M. F. M. Santana, F. J. Kroon, L. van Herwerden, G. Vamvounis and C. A. Motti, An assessment workflow to recover microplastics from complex biological matrices, Mar. Pollut. Bull., 2022, 179, 113676 CrossRef PubMed.
- S. Ducoli, M. Rani, C. Marchesi, S. Federici and L. E. Depero, in 2023 IEEE International Workshop on Metrology for the Sea; Learning to Measure Sea Health Parameters (MetroSea), IEEE, La Valletta, Malta, 2023, pp. 238–242 Search PubMed.
- C. Marchesi, M. Rani, S. Federici, I. Alessandri, I. Vassalini, S. Ducoli, L. Borgese, A. Zacco, A. Núñez-Delgado, E. Bontempi and L. E. Depero, Quantification of ternary microplastic mixtures through an ultra-compact near-infrared spectrometer coupled with chemometric tools, Environ. Res., 2023, 216, 114632 CrossRef CAS PubMed.
- M. T. Ekvall, M. Lundqvist, E. Kelpsiene, E. Šileikis, S. B. Gunnarsson and T. Cedervall, Nanoplastics formed during the mechanical breakdown of daily-use polystyrene products, Nanoscale Adv., 2019, 1, 1055–1061 RSC.
- C. Smith, S. Brown, N. Malone, S. Bevers, J. Ranville and D. H. Fairbrother, Nanoplastics prepared with uniformly distributed metal-tags: a novel approach to quantify size distribution and particle number concentration of polydisperse nanoplastics by single particle ICP-MS, Environ. Sci.: Nano, 2024, 11, 911–923 RSC.
- A. A. Cuthbertson, C. Lincoln, J. Miscall, L. M. Stanley, A. K. Maurya, A. S. Asundi, C. J. Tassone, N. A. Rorrer and G. T. Beckham, Characterization of polymer properties and identification of additives in commercially available research plastics, Green Chem., 2024, 26, 7067–7090 RSC.
- M. Davranche, C. Veclin, A.-C. Pierson-Wickmann, H. El Hadri, B. Grassl, L. Rowenczyk, A. Dia, A. Ter Halle, F. Blancho, S. Reynaud and J. Gigault, Are nanoplastics able to bind significant amount of metals? The lead example, Environ. Pollut., 2019, 249, 940–948 CrossRef CAS PubMed.
- M. Enfrin, J. Lee, Y. Gibert, F. Basheer, L. Kong and L. F. Dumée, Release of hazardous nanoplastic contaminants due to microplastics fragmentation under shear stress forces, J. Hazard. Mater., 2020, 384, 121393 CrossRef CAS PubMed.
- M. Ullmann, S. K. Friedlander and A. Schmidt-Ott, Nanoparticle Formation by Laser Ablation, J. Nanopart. Res., 2002, 4, 499–509 CrossRef CAS.
- S. Ravi-Kumar, B. Lies, H. Lyu and H. Qin, Laser Ablation of Polymers: A Review, Procedia Manuf., 2019, 34, 316–327 CrossRef.
- I. Elaboudi, S. Lazare, C. Belin, D. Talaga and C. Labrugère, From polymer films to organic nanoparticles suspensions by means of excimer laser ablation in water, Appl. Phys. A: Mater. Sci. Process., 2008, 93, 827–831 CrossRef CAS.
- D. E. Martínez-Tong, M. Sanz, T. A. Ezquerra, A. Nogales, J. F. Marco, M. Castillejo and E. Rebollar, Formation of polymer nanoparticles by UV pulsed laser ablation of poly (bisphenol A carbonate) in liquid environment, Appl. Surf. Sci., 2017, 418, 522–529 CrossRef.
- I. Kanehara, H. Yamashita, S. Fujii, T. Kimura, M. Yamamoto and T. Tanabe, Nano-Sized Polyethylene Particles Produced by Nano-Second UV Laser Ablation, Lasers Manuf. Mater. Process., 2023, 10, 389–399 CrossRef.
- I. Elaboudi, S. Lazare, C. Belin, D. Talaga and C. Labrugère, Underwater excimer laser ablation of polymers, Appl. Phys. A: Mater. Sci. Process., 2008, 92, 743–748 CrossRef CAS.
- A. Villacorta, L. Rubio, M. Alaraby, M. López-Mesas, V. Fuentes-Cebrian, O. H. Moriones, R. Marcos and A. Hernández, A new source of representative secondary PET nanoplastics. Obtention, characterization, and hazard evaluation, J. Hazard. Mater., 2022, 439, 129593 CrossRef CAS PubMed.
- K. Grigoriadi, M. Nooijens, A. Taşlı, M. Vanhouttem, S. Henke, L. Parker, J. Urbanus and A. Boersma, Experimental Validation of the Microplastic Index—Two Approaches to Understanding Microplastic Formation, Microplastics, 2023, 2, 350–370 CrossRef.
- A. Boersma, K. Grigoriadi, M. G. A. Nooijens, S. Henke, I. M. Kooter, L. A. Parker, A. Dortmans and J. H. Urbanus, Microplastic Index—How to Predict Microplastics Formation?, Polymers, 2023, 15, 2185 CrossRef CAS PubMed.
- A. Rebelein, I. Int-Veen, U. Kammann and J. P. Scharsack, Microplastic fibers — Underestimated threat to aquatic organisms?, Sci. Total Environ., 2021, 777, 146045 CrossRef CAS PubMed.
- R. Hufenus, F. A. Reifler, K. Maniura-Weber, A. Spierings and M. Zinn, Biodegradable Bicomponent Fibers from Renewable Sources: Melt-Spinning of Poly(lactic acid) and Poly[(3-hydroxybutyrate)-co-(3-hydroxyvalerate)], Macromol. Mater. Eng., 2012, 297, 75–84 CrossRef CAS.
- M. Schmiedgruber, R. Hufenus and D. M. Mitrano, Mechanistic understanding of microplastic fiber fate and sampling strategies: Synthesis and utility of metal doped polyester fibers, Water Res., 2019, 155, 423–430 CrossRef CAS PubMed.
- J. Xue, T. Wu, Y. Dai and Y. Xia, Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications, Chem. Rev., 2019, 119, 5298–5415 CrossRef CAS PubMed.
- A. Jemec, P. Horvat, U. Kunej, M. Bele and A. Kržan, Uptake and effects of microplastic textile fibers on freshwater crustacean Daphnia magna, Environ. Pollut., 2016, 219, 201–209 CrossRef CAS PubMed.
- Y. Liang, A. Lehmann, G. Yang, E. F. Leifheit and M. C. Rillig, Effects of Microplastic Fibers on Soil Aggregation and Enzyme Activities Are Organic Matter Dependent, Front. Environ. Sci., 2021, 9, 650155 CrossRef.
- M. Schmitt, K. Altmann, P. Fengler and M. Gehde, Air-based polyethylene fragmentation with high yield to form microplastic particles as reference material candidates, Appl. Res., 2024, 3, e202200121 CrossRef CAS.
- J. Hildebrandt and A. F. Thünemann, Aqueous Dispersions of Polypropylene: Toward Reference Materials for Characterizing Nanoplastics, Macromol. Rapid Commun., 2023, 2200874, 1–15 Search PubMed.
- S. Ducoli, M. Rani, C. Marchesi, M. Speziani, A. Zacco, G. Gavazzi, S. Federici and L. E. Depero, Comparison of different fragmentation techniques for the production of true-to-life microplastics, Talanta, 2025, 283, 127106 CrossRef CAS PubMed.
- L. Pessoni, C. Veclin, H. El Hadri, C. Cugnet, M. Davranche, A.-C. Pierson-Wickmann, J. Gigault, B. Grassl and S. Reynaud, Soap- and metal-free polystyrene latex particles as a nanoplastic model, Environ. Sci.: Nano, 2019, 6, 2253–2258 RSC.
- J. Jiménez-Lamana, L. Marigliano, J. Allouche, B. Grassl, J. Szpunar and S. Reynaud, A Novel Strategy for the Detection and Quantification of Nanoplastics by Single Particle Inductively Coupled Plasma Mass Spectrometry (ICP-MS), Anal. Chem., 2020, 92, 11664–11672 CrossRef PubMed.
- L. F. Muff, S. Balog, J. Adamcik, C. Weder and R. Lehner, Preparation of Well-Defined Fluorescent Nanoplastic Particles by Confined Impinging Jet Mixing, Environ. Sci. Technol., 2023, 57, 17201–17211 CrossRef CAS PubMed.
- M. Heinlaan, K. Kasemets, V. Aruoja, I. Blinova, O. Bondarenko, A. Lukjanova, A. Khosrovyan, I. Kurvet, M. Pullerits, M. Sihtmäe, G. Vasiliev, H. Vija and A. Kahru, Hazard evaluation of polystyrene nanoplastic with nine bioassays did not show particle-specific acute toxicity, Sci. Total Environ., 2020, 707, 136073 CrossRef CAS PubMed.
- O. Pikuda, E. G. Xu, D. Berk and N. Tufenkji, Toxicity Assessments of Micro- and Nanoplastics Can Be Confounded by Preservatives in Commercial Formulations, Environ. Sci. Technol. Lett., 2019, 6, 21–25 CrossRef CAS.
- M. Al-Sid-Cheikh, S. J. Rowland, R. Kaegi, T. B. Henry, M.-A. Cormier and R. C. Thompson, Synthesis of 14C-labelled polystyrene nanoplastics for environmental studies, Commun. Mater., 2020, 1, 97 CrossRef.
- D. M. Mitrano, A. Beltzung, S. Frehland, M. Schmiedgruber, A. Cingolani and F. Schmidt, Synthesis of metal-doped nanoplastics and their utility to investigate fate and behaviour in complex environmental systems, Nat. Nanotechnol., 2019, 14, 362–368 CrossRef CAS PubMed.
- R. J. Rauschendorfer, K. M. Whitham, S. Summer, S. A. Patrick, A. E. Pierce, H. Sefi-Cyr, S. Tadjiki, M. D. Kraft, S. R. Emory, D. A. Rider and M. D. Montaño, Development and Application of Nanoparticle-Nanopolymer Composite Spheres for the Study of Environmental Processes, Front. Toxicol., 2021, 3, 752296 CrossRef PubMed.
- Y. Lai, L. Dong, Q. Li, P. Li, Z. Hao, S. Yu and J. Liu, Counting Nanoplastics in Environmental Waters by Single Particle Inductively Coupled Plasma Mass Spectroscopy after Cloud-Point Extraction and In Situ Labeling of Gold Nanoparticles, Environ. Sci. Technol., 2021, 55, 4783–4791 CrossRef CAS PubMed.
- E. Grau, P.-Y. Dugas, J.-P. Broyer, C. Boisson, R. Spitz and V. Monteil, Aqueous Dispersions of Nonspherical Polyethylene Nanoparticles from Free-Radical Polymerization under Mild Conditions, Angew. Chem., 2010, 122, 6962–6964 CrossRef.
- G. Billuart, E. Bourgeat-Lami, M. Lansalot and V. Monteil, Free Radical Emulsion Polymerization of Ethylene, Macromolecules, 2014, 47, 6591–6600 CrossRef CAS.
- F. M. Bauers, R. Thomann and S. Mecking, Submicron Polyethylene Particles from Catalytic Emulsion Polymerization, J. Am. Chem. Soc., 2003, 125, 8838–8840 CrossRef CAS PubMed.
- P. Paik and K. K. Kar, High molecular weight polypropylene nanospheres: Synthesis and characterization, J. Appl. Polym. Sci., 2007, 105, 1133–1143 CrossRef CAS.
- G. Balakrishnan, M. Déniel, T. Nicolai, C. Chassenieux and F. Lagarde, Towards more realistic reference microplastics and nanoplastics: preparation of polyethylene micro/nanoparticles with a biosurfactant, Environ. Sci.: Nano, 2019, 6, 315–324 RSC.
- L. M. Johnson, J. B. Mecham, S. A. Krovi, M. M. M. Caffaro, S. Aravamudhan, A. L. Kovach, T. R. Fennell and N. P. Mortensen, Fabrication of polyethylene terephthalate (PET) nanoparticles with fluorescent tracers for studies in mammalian cells, Nanoscale Adv., 2021, 3, 339–346 RSC.
- P. Merdy, F. Delpy, A. Bonneau, S. Villain, L. Iordachescu, J. Vollertsen and Y. Lucas, Nanoplastic production procedure for scientific purposes: PP, PVC, PE-LD, PE-HD, and PS, Heliyon, 2023, 9, e18387 CrossRef CAS PubMed.
- K. Tanaka, Y. Takahashi, H. Kuramochi, M. Osako, S. Tanaka and G. Suzuki, Preparation of Nanoscale Particles of Five Major Polymers as Potential Standards for the Study of Nanoplastics, Small, 2021, 17, 2105781 CrossRef CAS PubMed.
- A. Robles-Martín, R. Amigot-Sánchez, L. Fernandez-Lopez, J. L. Gonzalez-Alfonso, S. Roda, V. Alcolea-Rodriguez, D. Heras-Márquez, D. Almendral, C. Coscolín, F. J. Plou, R. Portela, M. A. Bañares, Á. Martínez-del-Pozo, S. García-Linares, M. Ferrer and V. Guallar, Sub-micro- and nano-sized polyethylene terephthalate deconstruction with engineered protein nanopores, Nat. Catal., 2023, 6, 1174–1185 CrossRef.
- D. Cassano, R. La Spina, J. Ponti, I. Bianchi and D. Gilliland, Inorganic Species-Doped Polypropylene Nanoparticles for Multifunctional Detection, ACS Appl. Nano Mater., 2021, 4, 1551–1557 CrossRef CAS.
- A. G. Rodríguez-Hernández, J. A. Muñoz-Tabares, J. C. Aguilar-Guzmán and R. Vazquez-Duhalt, A novel and simple method for polyethylene terephthalate (PET) nanoparticle production, Environ. Sci.: Nano, 2019, 6, 2031–2036 RSC.
- J. Zhang, M. Peng, E. Lian, L. Xia, A. G. Asimakopoulos, S. Luo and L. Wang, Identification of Poly(ethylene terephthalate) Nanoplastics in Commercially Bottled Drinking Water Using Surface-Enhanced Raman Spectroscopy, Environ. Sci. Technol., 2023, 57, 8365–8372 CrossRef CAS PubMed.
- K. Y. Santizo, H. S. Mangold, Z. Mirzaei, H. Park, R. R. Kolan, G. Sarau, S. Kolle, T. Hansen, S. Christiansen and W. Wohlleben, Microplastic Materials for Inhalation Studies: Preparation by Solvent Precipitation and Comprehensive Characterization, Small, 2025, 21, 2405555 CrossRef CAS PubMed.
- W. Saad and R. Prud'homme, Principles of nanoparticle formation by Flash Nanoprecipitation, Nano Today, 2016, 11, 212–227, DOI:10.1016/j.nantod.2016.04.006.
- B. Yang, L. Xu, Y. Liu, B. Liu and M. Zhang, Preparation of monodisperse polystyrene microspheres with different functional groups using soap-free emulsion polymerization, Colloid Polym. Sci., 2021, 299, 1095–1102 CrossRef CAS.
- D. Tatsii, S. Bucci, T. Bhowmick, J. Guettler, L. Bakels, G. Bagheri and A. Stohl, Shape Matters: Long-Range Transport of Microplastic Fibers in the Atmosphere, Environ. Sci. Technol., 2024, 58, 671–682 CrossRef CAS PubMed.
- S. Bhargava, J. J. H. Chu and S. Valiyaveettil, Controlled Dye Aggregation in Sodium Dodecylsulfate-Stabilized Poly(methylmethacrylate) Nanoparticles as Fluorescent Imaging Probes, ACS Omega, 2018, 3, 7663–7672 CrossRef CAS PubMed.
- J. Rao and K. Geckeler, Polymer Nanoparticles: Preparation Techniques and Size-Control Parameters, Prog. Polym. Sci., 2011, 36, 887–913, DOI:10.1016/j.progpolymsci.2011.01.001.
- J. R. Peller, S. P. Mezyk, S. Shidler, J. Castleman, S. Kaiser, R. F. Faulkner, C. D. Pilgrim, A. Wilson, S. Martens and G. P. Horne, Facile nanoplastics formation from macro and microplastics in aqueous media, Environ. Pollut., 2022, 313, 120171 CrossRef CAS PubMed.
- Y. Wang, R. Deng, L. Yang and C. D. Bain, Fabrication of monolayers of uniform polymeric particles by inkjet printing of monodisperse emulsions produced by microfluidics, Lab Chip, 2019, 19, 3077–3085 RSC.
- L. Sgier, R. Freimann, A. Zupanic and A. Kroll, Flow cytometry combined with viSNE for the analysis of microbial biofilms and detection of microplastics, Nat. Commun., 2016, 7, 11587 CrossRef CAS PubMed.
- F. Ribeiro, A. Duarte and J. Da Costa, Staining methodologies for microplastics screening, TrAC, Trends Anal. Chem., 2024, 172, 117555 CrossRef CAS.
- S. Liu, E. Shang, J. Liu, Y. Wang, N. Bolan, M. B. Kirkham and Y. Li, What have we known so far for fluorescence staining and quantification of microplastics: A tutorial review, Front. Environ. Sci. Eng., 2022, 16, 8 Search PubMed.
- Z. Gao, K. Wontor and J. V. Cizdziel, Labeling Microplastics with Fluorescent Dyes for, Recovery, and Degradation ExperimentsDetection, Molecules, 2022, 27, 7415 Search PubMed.
- W. J. Shim, Y. K. Song, S. H. Hong and M. Jang, Identification and quantification of microplastics using Nile Red staining, Mar. Pollut. Bull., 2016, 113, 469–476 Search PubMed.
- G. Erni-Cassola, M. I. Gibson, R. C. Thompson and J. A. Christie-Oleza, Lost, but Found with Nile Red: A Novel Method for Detecting and Quantifying Small Microplastics (1 mm to 20 μm) in Environmental Samples, Environ. Sci. Technol., 2017, 51, 13641–13648 CrossRef CAS PubMed.
- V. Caponetti, A. Mavridi-Printezi, M. Cingolani, E. Rampazzo, D. Genovese, L. Prodi, D. Fabbri and M. Montalti, A Selective Ratiometric Fluorescent Probe for No-Wash Detection of PVC Microplastic, Polymers, 2021, 13, 1588 Search PubMed.
- T. Behnke, C. Würth, E.-M. Laux, K. Hoffmann and U. Resch-Genger, Simple strategies towards bright polymer particles via one-step staining procedures, Dyes Pigm., 2012, 94(2), 247–257 CrossRef CAS.
- N. Yakovenko, B. Amouroux, M. Albignac, F. Collin, C. Roux, A.-F. Mingotaud, P. Roblin, C. Coudret and A. ter Halle, Top-down synthesis of luminescent microplastics and nanoplastics by incorporation of upconverting nanoparticles for environmental assessment, Environ. Sci.: Nano, 2022, 9, 2453–2463 RSC.
- M. T. Sturm, E. Myers, D. Schober, A. Korzin and K. Schuhen, Development of an Inexpensive and Comparable Microplastic Detection Method Using Fluorescent Staining with Novel Nile Red Derivatives, Analytica, 2023, 4, 27–44 CrossRef CAS.
- J. Zhang, H. Li, Y. Li, S. Li, Y. Xu and H. Li, Boron-doped carbon nanoparticles for identification and tracing of microplastics in “Turn-on” fluorescence mode, Chem. Eng. J., 2022, 435, 135075 CrossRef CAS.
- L. Marigliano, B. Grassl, J. Szpunar, S. Reynaud and J. Jiménez-Lamana, Nanoplastic Labelling with Metal Probes: Analytical Strategies for Their Sensitive Detection and Quantification by ICP Mass Spectrometry, Molecules, 2021, 26, 7093 CrossRef CAS PubMed.
- L. Hildebrandt, F. Nack, T. Zimmermann and D. Pröfrock, Microplastics as a Trojan horse for trace metals, J. Hazard. Mater. Lett., 2021, 2, 100035, DOI:10.1016/j.hazl.2021.100035.
- R. A. Ramli, W. A. Laftah and S. Hashim, Core–shell polymers: a review, RSC Adv., 2013, 3, 15543–15565 RSC.
- W. Kaminsky, in Handbook of Polymer Synthesis, CRC Press, 2nd edn, 2004 Search PubMed.
- E. L. Tran, S. Bevers, C. Smith, S. Brown, N. Malone, D. H. Fairbrother and J. F. Ranville, Use of metal-tagged environmentally representative micro- and nanoplastic particles to investigate transport and retention through porous media using single particle ICP-MS, Microplast. Nanoplast., 2024, 4, 10 CrossRef.
- Y. Luo, L. Li, Y. Feng, R. Li, J. Yang, W. J. G. M. Peijnenburg and C. Tu, Quantitative tracing of uptake and transport of submicrometre plastics in crop plants using lanthanide chelates as a dual-functional tracer, Nat. Nanotechnol., 2022, 17, 424–431 CrossRef CAS PubMed.
- S. J. Taipale, E. Peltomaa, J. V. K. Kukkonen, M. J. Kainz, P. Kautonen and M. Tiirola, Tracing the fate of microplastic carbon in the aquatic food web by compound-specific isotope analysis, Sci. Rep., 2019, 9, 19894 CrossRef CAS PubMed.
- S. J. Taipale, J. Vesamäki, P. Kautonen, J. V. K. Kukkonen, C. Biasi, R. Nissinen and M. Tiirola, Biodegradation of microplastic in freshwaters: A long-lasting process affected by the lake microbiome, Environ. Microbiol., 2023, 25, 2669–2680 CrossRef CAS PubMed.
- Y. Liu, J. Li, B. V. Parakhonskiy, R. Hoogenboom, A. Skirtach and S. De Neve, Labelling of micro- and nanoplastics for environmental studies: state-of-the-art and future challenges, J. Hazard. Mater., 2024, 462, 132785 CrossRef CAS PubMed.
- M. Sander, H.-P. E. Kohler and K. McNeill, Assessing the environmental transformation of nanoplastic through 13C-labelled polymers, Nat. Nanotechnol., 2019, 14, 301–303 CrossRef CAS PubMed.
- A. Mauel, B. Pötzschner, N. Meides, R. Siegel, P. Strohriegl and J. Senker, Quantification of photooxidative defects in weathered microplastics using 13 C multiCP NMR spectroscopy, RSC Adv., 2022, 12, 10875–10885 RSC.
- I. Goßmann, K. Mattsson, M. Hassellöv, C. Crazzolara, A. Held, T.-B. Robinson, O. Wurl and B. M. Scholz-Böttcher, Unraveling the Marine Microplastic Cycle: The First Simultaneous Data Set for Air, Sea Surface Microlayer, and Underlying Water, Environ. Sci. Technol., 2023, 57, 16541–16551 CrossRef PubMed.
- T. Lauschke, G. Dierkes, P. Schweyen and T. A. Ternes, Evaluation of poly(styrene-d5) and poly(4-fluorostyrene) as internal standards for microplastics quantification by thermoanalytical methods, J. Anal. Appl. Pyrolysis, 2021, 159, 105310 CrossRef CAS.
- E. D. Okoffo, B. J. Tscharke, J. W. O'Brien, S. O'Brien, F. Ribeiro, S. D. Burrows, P. M. Choi, X. Wang, J. F. Mueller and K. V. Thomas, Release of Plastics to Australian Land from Biosolids End-Use, Environ. Sci. Technol., 2020, 54, 15132–15141 CrossRef CAS PubMed.
- M. T. Zumstein, A. Schintlmeister, T. F. Nelson, R. Baumgartner, D. Woebken, M. Wagner, H.-P. E. Kohler, K. McNeill and M. Sander, Biodegradation of synthetic polymers in soils: Tracking carbon into CO 2 and microbial biomass, Sci. Adv., 2018, 4, eaas9024 CrossRef CAS PubMed.
- M. Goudriaan, V. H. Morales, M. T. J. van der Meer, A. Mets, R. T. Ndhlovu, J. van Heerwaarden, S. Simon, V. B. Heuer, K.-U. Hinrichs and H. Niemann, A stable isotope assay with 13C-labeled polyethylene to investigate plastic mineralization mediated by Rhodococcus ruber, Mar. Pollut. Bull., 2023, 186, 114369 CrossRef CAS PubMed.
- M. T. Zumstein, R. Narayan, H.-P. E. Kohler, K. McNeill and M. Sander, Dos and Do Nots When Assessing the Biodegradation of Plastics, Environ. Sci. Technol., 2019, 53, 9967–9969 CrossRef CAS PubMed.
- C. Im, H. Kim, J. Zaheer, J. Y. Kim, Y.-J. Lee, C. M. Kang and J. S. Kim, PET Tracing of Biodistribution for Orally Administered 64 Cu-Labeled Polystyrene in Mice, J. Nucl. Med., 2022, 63, 461–467 CrossRef CAS PubMed.
- M. Munir, M. Subechi, A. Nurmanjaya, K. E. Prasetya, F. Rindiyantono, Chairuman, C. Pratama, Yanto, A. Pujiyanto, H. Setiawan, D. A. Sarwono, E. Sarmini, M. E. Fara and H. Suseno, Development of a polystyrene-based microplastic model for bioaccumulation and biodistribution study using radiotracing and nuclear analysis method, Mar. Pollut. Bull., 2024, 201, 116283 CrossRef CAS PubMed.
- A. Stricker, S. Hilpmann, A. Mansel, K. Franke and S. Schymura, Radiolabeling of Micro-/Nanoplastics via In-Diffusion, Nanomaterials, 2023, 13, 2687 CrossRef CAS PubMed.
- M. Munir, U. N. Sholikhah, E. Lestari, A. Pujiyanto, K. E. Prasetya, A. Nurmanjaya, Yanto, D. A. Sarwono, M. Subechi and H. Suseno, Iodine-131 radiolabeled polyvinylchloride: A potential radiotracer for micro and nanoplastics bioaccumulation and biodistribution study in organisms, Mar. Pollut. Bull., 2023, 188, 114627 CrossRef CAS PubMed.
- M. Al-Sid-Cheikh, S. J. Rowland, K. Stevenson, C. Rouleau, T. B. Henry and R. C. Thompson, Uptake, Whole-Body Distribution, and Depuration of Nanoplastics by the Scallop Pecten maximus at Environmentally Realistic Concentrations, Environ. Sci. Technol., 2018, 52, 14480–14486 CrossRef CAS PubMed.
- L. Tian, Q. Chen, W. Jiang, L. Wang, H. Xie, N. Kalogerakis, Y. Ma and R. Ji, A carbon-14 radiotracer-based study on the phototransformation of polystyrene nanoplastics in water versus in air, Environ. Sci.: Nano, 2019, 6, 2907–2917 RSC.
- P. J. Gawne, F. Man, P. J. Blower and R. T. M. de Rosales, Direct Cell Radiolabeling for in Vivo Cell Tracking with PET and SPECT Imaging, Chem. Rev., 2022, 122, 10266–10318 CrossRef CAS PubMed.
- W. Dai, J. Zhang, Y. Wang, C. Jiao, Z. Song, Y. Ma, Y. Ding, Z. Zhang and X. He, Radiolabeling of Nanomaterials: Advantages and Challenges, Front. Toxicol., 2021, 3, 753316 CrossRef PubMed.
- C. M. Lanctôt, M. Al-Sid-Cheikh, A. I. Catarino, T. Cresswell, B. Danis, H. K. Karapanagioti, T. Mincer, F. Oberhänsli, P. Swarzenski, I. Tolosa and M. Metian, Application of nuclear techniques to environmental plastics research, J. Environ. Radioact., 2018, 192, 368–375 CrossRef PubMed.
- O. S. Alimi, D. Claveau-Mallet, R. S. Kurusu, M. Lapointe, S. Bayen and N. Tufenkji, Weathering pathways and protocols for environmentally relevant microplastics and nanoplastics: What are we missing?, J. Hazard. Mater., 2022, 423, 126955 CrossRef CAS PubMed.
- P. Liu, Y. Shi, X. Wu, H. Wang, H. Huang, X. Guo and S. Gao, Review of the artificially-accelerated aging technology and ecological risk of microplastics, Sci. Total Environ., 2021, 768, 144969 CrossRef CAS PubMed.
- L. Liu, M. Xu, Y. Ye and B. Zhang, On the degradation of (micro)plastics: Degradation methods, influencing factors, environmental impacts, Sci. Total Environ., 2022, 806, 151312 CrossRef CAS PubMed.
- J. E. Pickett, in Handbook of Environmental Degradation of Materials, Elsevier, 2018, pp. 163–184 Search PubMed.
- J. Duan, N. Bolan, Y. Li, S. Ding, T. Atugoda, M. Vithanage, B. Sarkar, D. C. W. Tsang and M. B. Kirkham, Weathering of microplastics and interaction with other coexisting constituents in terrestrial and aquatic environments, Water Res., 2021, 196, 117011 CrossRef CAS PubMed.
- G. Binda, G. Kalčíková, I. J. Allan, R. Hurley, E. Rødland, D. Spanu and L. Nizzetto, Microplastic aging processes: Environmental relevance and analytical implications, TrAC, Trends Anal. Chem., 2024, 172, 117566 CrossRef CAS.
- C. D. Rummel, A. Jahnke, E. Gorokhova, D. Kühnel and M. Schmitt-Jansen, Impacts of Biofilm Formation on the Fate and Potential Effects of Microplastic in the Aquatic Environment, Environ. Sci. Technol. Lett., 2017, 4, 258–267 CrossRef CAS.
- R. B. Schefer, A. Armanious and D. M. Mitrano, Eco-Corona Formation on Plastics: Adsorption of Dissolved Organic Matter to Pristine and Photochemically Weathered Polymer Surfaces, Environ. Sci. Technol., 2023, 57, 14707–14716 CrossRef CAS PubMed.
- A. Andrady, S. Hamid, X. Hu and A. Torikai, Effects of increased solar ultraviolet radiation on materials, J. Photochem. Photobiol., B, 1998, 46, 96–103 CrossRef CAS PubMed.
- A. Chamas, H. Moon, J. Zheng, Y. Qiu, T. Tabassum, J. H. Jang, M. Abu-Omar, S. L. Scott and S. Suh, Degradation Rates of Plastics in the Environment, ACS Sustainable Chem. Eng., 2020, 8, 3494–3511 CrossRef CAS.
- K. G. de Castro Monsores, A. O. da Silva, S. de Sant’ Ana Oliveira, R. P. Weber, P. F. Filho and S. N. Monteiro, Influence of ultraviolet radiation on polystyrene, J. Mater. Res. Technol., 2021, 13, 359–365 CrossRef CAS.
- Y. K. Song, S. H. Hong, M. Jang, G. M. Han, S. W. Jung and W. J. Shim, Combined Effects of UV Exposure Duration and Mechanical Abrasion on Microplastic Fragmentation by Polymer Type, Environ. Sci. Technol., 2017, 51, 4368–4376 CrossRef CAS PubMed.
- L. M. Hernandez, J. Grant, P. S. Fard, J. M. Farner and N. Tufenkji, Analysis of ultraviolet and thermal degradations of four common microplastics and evidence of nanoparticle release, J. Hazard. Mater. Lett., 2023, 4, 100078 CrossRef CAS.
- J. Reineccius, M. Schönke and J. J. Waniek, Abiotic Long-Term Simulation of Microplastic Weathering Pathways under Different Aqueous Conditions, Environ. Sci. Technol., 2023, 57, 963–975 CrossRef CAS PubMed.
- J. E. Bullard, Z. Zhou, S. Davis and S. Fowler, Breakdown and Modification of Microplastic Beads by Aeolian Abrasion, Environ. Sci. Technol., 2023, 57, 76–84 CrossRef CAS PubMed.
- P. L. Corcoran, in Handbook of Microplastics in the Environment, ed. T. Rocha-Santos, M. F. Costa and C. Mouneyrac, Springer International Publishing, Cham, 2022, pp. 531–542 Search PubMed.
- E. Rahman, S. BinAhmed, P. Keyes, C. Alberg, S. Godfreey-Igwe, G. Haugstad and B. Xiong, Nanoscale Abrasive Wear of Polyethylene: A Novel Approach To Probe Nanoplastic Release at the Single Asperity Level, Environ. Sci. Technol., 2024, 58, 13845–13855 CrossRef CAS PubMed.
- C. Schür, S. Zipp, T. Thalau and M. Wagner, Microplastics but not natural particles induce multigenerational effects in Daphnia magna, Environ. Pollut., 2020, 260, 113904 CrossRef PubMed.
- G. Binda, G. Zanetti, A. Bellasi, D. Spanu, G. Boldrocchi, R. Bettinetti, A. Pozzi and L. Nizzetto, Physicochemical and biological ageing processes of (micro)plastics in the environment: a multi-tiered study on polyethylene, Environ. Sci. Pollut. Res., 2023, 30, 6298–6312 CrossRef CAS PubMed.
- J. Gregory, Particles in Water: Properties and Processes, Particles in Water, CRC Press, 1st edn, 2005 Search PubMed.
- K. A. Jensen, Y. Kembouche, E. Christiansen, N. Jacobsen, H. Wallin, C. Guiot, O. Spalla and O. Witschger, Final protocol for producing suitable manufactured nanomaterial exposure media, NANOGENOTOX deliverable report.
- B. Annangi, A. Villacorta, L. Vela, A. Tavakolpournegari, R. Marcos and A. Hernández, Effects of true-to-life PET nanoplastics using primary human nasal epithelial cells, Environ. Toxicol. Pharmacol., 2023, 100, 104140 CrossRef CAS PubMed.
- J. Seghers, M. Günther, A. Breidbach, N. Peez, W. Imhof and H. Emteborg, Feasibility of using quantitative 1H-NMR spectroscopy and ultra-microbalances for investigation of a PET microplastic reference material, Anal. Bioanal. Chem., 2023, 415, 3033–3040 CrossRef CAS PubMed.
- E. Martínez-Francés, B. van Bavel, R. Hurley, L. Nizzetto, S. Pakhomova, N. T. Buenaventura, C. Singdahl-Larsen, M.-L. T. Magni, J. E. Johansen and A. Lusher, Innovative reference materials for method validation in microplastic analysis including interlaboratory comparison exercises, Anal. Bioanal. Chem., 2023, 415, 2907–2919, DOI:10.1007/s00216-023-04636-4.
- M. Matsueda, M. Mattonai, I. Iwai, A. Watanabe, N. Teramae, W. Robberson, H. Ohtani, Y.-M. Kim and C. Watanabe, Preparation and test of a reference mixture of eleven polymers with deactivated inorganic diluent for microplastics analysis by pyrolysis-GC–MS, J. Anal. Appl. Pyrolysis, 2021, 154, 104993 CrossRef CAS.
- J. M. Hankett, J. L. Holtz, I. Walker-Franklin, K. Shaffer, J. Jourdan, D. C. Batiste, J. M. Garcia, C. Kaczan, W. Wohlleben and L. Ferguson, Matrix Matters: novel insights for the extraction, preparation, and quantitation of microplastics in a freshwater mesocosm study, Microplast. Nanoplast., 2023, 3, 13 CrossRef.
- T. Ishimura, I. Iwai, K. Matsui, M. Mattonai, A. Watanabe, W. Robberson, A.-M. Cook, H. L. Allen, W. Pipkin, N. Teramae, H. Ohtani and C. Watanabe, Qualitative and quantitative analysis of mixtures of microplastics in the presence of calcium carbonate by pyrolysis-GC/MS, J. Anal. Appl. Pyrolysis, 2021, 157, 105188 CrossRef CAS.
- A. B. Silva, A. S. Bastos, C. I. L. Justino, J. P. da Costa, A. C. Duarte and T. A. P. Rocha-Santos, Microplastics in the environment: Challenges in analytical chemistry - A review, Anal. Chim. Acta, 2018, 1017, 1–19 CrossRef CAS PubMed.
- S. Huppertsberg and T. P. Knepper, Instrumental analysis of microplastics—benefits and challenges, Anal. Bioanal. Chem., 2018, 410, 6343–6352 CrossRef CAS PubMed.
- S. Primpke, S. H. Christiansen, W. Cowger, H. D. Frond, A. Deshpande, M. Fischer, E. B. Holland, M. Meyns, B. A. O'Donnell, B. E. Ossmann, M. Pittroff, G. Sarau, B. M. Scholz-Böttcher and K. J. Wiggin, Critical Assessment of Analytical Methods for the Harmonized and Cost-Efficient Analysis of Microplastics, Appl. Spectrosc., 2020, 74, 1012–1047 CrossRef CAS PubMed.
- S. Choi, S. Lee, M.-K. Kim, E.-S. Yu and Y.-S. Ryu, Challenges and Recent Analytical Advances in Micro/Nanoplastic Detection, Anal. Chem., 2024, 96, 8846–8854 CrossRef CAS PubMed.
- W. Fu, J. Min, W. Jiang, Y. Li and W. Zhang, Separation, characterization and identification of microplastics and nanoplastics in the environment, Sci. Total Environ., 2020, 721, 137561 CrossRef CAS PubMed.
- V. S. Fringer, L. P. Fawcett, D. M. Mitrano and M. A. Maurer-Jones, Impacts of Nanoplastics on the Viability and Riboflavin Secretion in the Model Bacteria Shewanella oneidensis, Front. Environ. Sci., 2020, 8, 97 CrossRef.
- I. Demir-Yilmaz, N. Yakovenko, C. Roux, P. Guiraud, F. Collin, C. Coudret, A. ter Halle and C. Formosa-Dague, The role of microplastics in microalgae cells aggregation: A study at the molecular scale using atomic force microscopy, Sci. Total Environ., 2022, 832, 155036 CrossRef CAS PubMed.
- M. Holzer, D. M. Mitrano, L. Carles, B. Wagner and A. Tlili, Important ecological processes are affected by the accumulation and trophic transfer of nanoplastics in a freshwater periphyton-grazer food chain, Environ. Sci.: Nano, 2022, 9, 2990–3003 RSC.
- P. E. Redondo-Hasselerharm, G. Vink, D. M. Mitrano and A. A. Koelmans, Metal-doping of nanoplastics enables accurate assessment of uptake and effects on Gammarus pulex, Environ. Sci.: Nano, 2021, 8, 1761–1770 RSC.
- Y. Tan, X. Zhu, D. Wu, E. Song and Y. Song, Compromised Autophagic Effect of Polystyrene Nanoplastics Mediated by Protein Corona Was Recovered after Lysosomal Degradation of Corona, Environ. Sci. Technol., 2020, 54, 11485–11493 CrossRef CAS PubMed.
- Y. Zhao, R. Qiao, S. Zhang and G. Wang, Metabolomic profiling reveals the intestinal toxicity of different length of microplastic fibers on zebrafish (Danio rerio), J. Hazard. Mater., 2021, 403, 123663 CrossRef CAS PubMed.
- M. Cole, R. Coppock, P. K. Lindeque, D. Altin, S. Reed, D. W. Pond, L. Sørensen, T. S. Galloway and A. M. Booth, Effects of Nylon Microplastic on Feeding, Lipid Accumulation, and Moulting in a Coldwater Copepod, Environ. Sci. Technol., 2019, 53, 7075–7082 CrossRef CAS PubMed.
- R. Qiao, Y. Deng, S. Zhang, M. B. Wolosker, Q. Zhu, H. Ren and Y. Zhang, Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish, Chemosphere, 2019, 236, 124334 CrossRef CAS PubMed.
- M. Cole, C. Liddle, G. Consolandi, C. Drago, C. Hird, P. K. Lindeque and T. S. Galloway, Microplastics, microfibres and nanoplastics cause variable sub-lethal responses in mussels (Mytilus spp.), Mar. Pollut. Bull., 2020, 160, 111552 CrossRef CAS PubMed.
- S. Ziajahromi, A. Kumar, P. A. Neale and F. D. L. Leusch, Impact of Microplastic Beads and Fibers on Waterflea (Ceriodaphnia dubia) Survival, Growth, and Reproduction: Implications of Single and Mixture Exposures, Environ. Sci. Technol., 2017, 51, 13397–13406 CrossRef CAS PubMed.
- Y. Zhang, J. Ju, X. Long, M. Zhu, Y. Jiang and H. Yang, Length-dependent toxic effects of microplastic fibers on Chlorella pyrenoidosa, Environ. Pollut., 2024, 342, 123037 CrossRef CAS PubMed.
- M. Tamayo-Belda, A. V. Pérez-Olivares, G. Pulido-Reyes, K. Martin-Betancor, M. González-Pleiter, F. Leganés, D. M. Mitrano, R. Rosal and F. Fernández-Piñas, Tracking nanoplastics in freshwater microcosms and their impacts to aquatic organisms, J. Hazard. Mater., 2023, 445, 130625 CrossRef CAS PubMed.
- A. E. Rubin, R. Gnaim, S. Levi and I. Zucker, Risk assessment framework for microplastic in marine environments, Sci. Total Environ., 2023, 901, 166459 CrossRef CAS PubMed.
- M. Heinlaan, K. Kasemets, V. Aruoja, I. Blinova, O. Bondarenko, A. Lukjanova, A. Khosrovyan, I. Kurvet, M. Pullerits, M. Sihtmäe, G. Vasiliev, H. Vija and A. Kahru, Hazard evaluation of polystyrene nanoplastic with nine bioassays did not show particle-specific acute toxicity, Sci. Total Environ., 2020, 707, 136073 CrossRef CAS PubMed.
- X. Zhou, G. Wang, X. An, J. Wu, K. Fan, L. Xu, C. Li and Y. Xue, Polystyrene microplastic particles: In vivo and in vitro ocular surface toxicity assessment, Environ. Pollut., 2022, 303, 119126 CrossRef CAS PubMed.
- S. Song, F. van Dijk, G. F. Vasse, Q. Liu, I. F. Gosselink, E. Weltjens, A. H. V. Remels, M. H. de Jager, S. Bos, C. Li, T. Stoeger, M. Rehberg, D. Kutschke, G. W. A. van Eck, X. Wu, S. H. Willems, D. H. A. Boom, I. M. Kooter, D. Spierings, R. Wardenaar, M. Cole, M. C. Nawijn, A. Salvati, R. Gosens and B. N. Melgert, Inhalable Textile Microplastic Fibers Impair Airway Epithelial Differentiation, Am. J. Respir. Crit. Care Med., 2024, 209, 427–443 CrossRef CAS PubMed.
- L. Ma, Z. Wu, Z. Lu, L. Yan, X. Dong, Z. Dai, R. Sun, P. Hong, C. Zhou and C. Li, Differences in toxicity induced by the various polymer types of nanoplastics on HepG2 cells, Sci. Total Environ., 2024, 918, 170664 CrossRef CAS PubMed.
- S. Han, J. Bang, D. Choi, J. Hwang, T. Kim, Y. Oh, Y. Hwang, J. Choi and J. Hong, Surface Pattern Analysis of Microplastics and Their Impact on Human-Derived Cells, ACS Appl. Polym. Mater., 2020, 2, 4541–4550 CrossRef CAS.
- D. Choi, J. Hwang, J. Bang, S. Han, T. Kim, Y. Oh, Y. Hwang, J. Choi and J. Hong, In vitro toxicity from a physical perspective of polyethylene microplastics based on statistical curvature change analysis, Sci. Total Environ., 2021, 752, 142242 CrossRef CAS PubMed.
- A. Villacorta, L. Vela, M. Morataya-Reyes, R. Llorens-Chiralt, L. Rubio, M. Alaraby, R. Marcos and A. Hernández, Titanium-doped PET nanoplastics of environmental origin as a true-to-life model of nanoplastic, Sci. Total Environ., 2023, 880, 163151 CrossRef CAS PubMed.
- W. A. Da Silva Brito, D. Singer, L. Miebach, F. Saadati, K. Wende, A. Schmidt and S. Bekeschus, Comprehensive in vitro polymer type, concentration, and size correlation analysis to microplastic toxicity and inflammation, Sci. Total Environ., 2023, 854, 158731 CrossRef CAS PubMed.
- G. M. DeLoid, X. Cao, D. Bitounis, D. Singh, P. M. Llopis, B. Buckley and P. Demokritou, Toxicity, uptake, and nuclear translocation of ingested micro-nanoplastics in an in vitro model of the small intestinal epithelium, Food Chem. Toxicol., 2021, 158, 112609 Search PubMed.
- S. Jeon, J. Clavadetscher, D.-K. Lee, S. V. Chankeshwara, M. Bradley and W.-S. Cho, Surface Charge-Dependent Cellular Uptake of Polystyrene Nanoparticles, Nanomaterials, 2018, 8, 1028 Search PubMed.
- N. J. Clark, F. R. Khan, C. Crowther, D. M. Mitrano and R. C. Thompson, Uptake, distribution and elimination of palladium-doped polystyrene nanoplastics in rainbow trout (Oncorhynchus mykiss) following dietary exposure, Sci. Total Environ., 2023, 854, 158765 CrossRef CAS PubMed.
- F. Ribeiro, D. M. Mitrano, C. Hacker, P. Cherek, K. Brigden, S. L. Kaserzon, K. V. Thomas and T. S. Galloway, Short Depuration of Oysters Intended for Human Consumption Is Effective at Reducing Exposure to Nanoplastics, Environ. Sci. Technol., 2022, 56, 16716–16725 Search PubMed.
- J. E. Ward, S. Zhao, B. A. Holohan, K. M. Mladinich, T. W. Griffin, J. Wozniak and S. E. Shumway, Selective Ingestion and Egestion of Plastic Particles by the Blue Mussel (Mytilus edulis) and Eastern Oyster (Crassostrea virginica): Implications for Using Bivalves as Bioindicators of Microplastic Pollution, Environ. Sci. Technol., 2019, 53, 8776–8784 CrossRef CAS PubMed.
- M. Rosa, J. E. Ward, S. E. Shumway, G. H. Wikfors, E. Pales-Espinosa and B. Allam, Effects of particle surface properties on feeding selectivity in the eastern oyster Crassostrea virginica and the blue mussel Mytilus edulis, J. Exp. Mar. Biol. Ecol., 2013, 446, 320–327 CrossRef.
- E. Lahive, R. Cross, A. I. Saarloos, A. A. Horton, C. Svendsen, R. Hufenus and D. M. Mitrano, Earthworms ingest microplastic fibres and nanoplastics with effects on egestion rate and long-term retention, Sci. Total Environ., 2022, 807, 151022 CrossRef CAS PubMed.
- W. M. Heinze, D. M. Mitrano, E. Lahive, J. Koestel and G. Cornelis, Nanoplastic Transport in Soil via Bioturbation by Lumbricus terrestris, Environ. Sci. Technol., 2021, 55, 16423–16433 CrossRef CAS PubMed.
- A. S. Keller, J. Jimenez-Martinez and D. M. Mitrano, Transport of Nano- and Microplastic through Unsaturated Porous Media from Sewage Sludge Application, Environ. Sci. Technol., 2020, 54, 911–920 CrossRef CAS PubMed.
- D. Shaniv, I. Dror and B. Berkowitz, Effects of particle size and surface chemistry on plastic nanoparticle transport in saturated natural porous media, Chemosphere, 2021, 262, 127854 CrossRef CAS PubMed.
- G. Pulido-Reyes, L. Magherini, C. Bianco, R. Sethi, U. von Gunten, R. Kaegi and D. M. Mitrano, Nanoplastics removal during drinking water treatment: Laboratory- and pilot-scale experiments and modeling, J. Hazard. Mater., 2022, 436, 129011 CrossRef CAS PubMed.
- S. Frehland, R. Kaegi, R. Hufenus and D. M. Mitrano, Long-term assessment of nanoplastic particle and microplastic fiber flux through a pilot wastewater treatment plant using metal-doped plastics, Water Res., 2020, 182, 115860 Search PubMed.
- L. Sørensen, A. S. Groven, I. A. Hovsbakken, O. Del Puerto, D. F. Krause, A. Sarno and A. M. Booth, UV degradation of natural and synthetic microfibers causes fragmentation and release of polymer degradation products and chemical additives, Sci. Total Environ., 2021, 755, 143170 Search PubMed.
- S. T. L. Sait, L. Sørensen, S. Kubowicz, K. Vike-Jonas, S. V. Gonzalez, A. G. Asimakopoulos and A. M. Booth, Microplastic fibres from synthetic textiles: Environmental degradation and additive chemical content, Environ. Pollut., 2021, 268, 115745 CrossRef CAS PubMed.
- M. N. Miranda, M. J. Sampaio, P. B. Tavares, A. M. T. Silva and M. F. R. Pereira, Aging assessment of microplastics (LDPE, PET and uPVC) under urban environment stressors, Sci. Total Environ., 2021, 796, 148914 CrossRef CAS PubMed.
- C. Harb, N. Pokhrel and H. Foroutan, Quantification of the Emission of Atmospheric Microplastics and Nanoplastics via Sea Spray, Environ. Sci. Technol. Lett., 2023, 10, 513–519 Search PubMed.
- M. Cingolani, E. Rampazzo, N. Zaccheroni, D. Genovese and L. Prodi, Fluorogenic hyaluronan nanogels for detection of micro- and nanoplastics in water, Environ. Sci.: Nano, 2022, 9, 582–588 RSC.
- A. Shorny, F. Steiner, H. Hörner and S. M. Skoff, Imaging and identification of single nanoplastic particles and agglomerates, Sci. Rep., 2023, 13, 10275 CrossRef CAS PubMed.
- B. Chaisrikhwun, S. Ekgasit and P. Pienpinijtham, Size-independent quantification of nanoplastics in various aqueous media using surfaced-enhanced Raman scattering, J. Hazard. Mater., 2023, 442, 130046 CrossRef CAS PubMed.
- H. Cai, M. Chen, F. Du, S. Matthews and H. Shi, Separation and enrichment of nanoplastics in environmental water samples via ultracentrifugation, Water Res., 2021, 203, 117509 CrossRef CAS PubMed.
- L. Chang, S. Jiang, J. Luo, J. Zhang, X. Liu, C.-Y. Lee and W. Zhang, Nanowell-enhanced Raman spectroscopy enables the visualization and quantification of nanoplastics in the environment, Environ. Sci.: Nano, 2022, 9, 542–553 RSC.
- A. Moraz and F. Breider, Detection and Quantification of Nonlabeled Polystyrene Nanoparticles Using a Fluorescent Molecular Rotor, Anal. Chem., 2021, 93, 14976–14984 Search PubMed.
- A. H. Tophinke, A. Joshi, U. Baier, R. Hufenus and D. M. Mitrano, Systematic development of extraction methods for quantitative microplastics analysis in soils using metal-doped plastics, Environ. Pollut., 2022, 311, 119933 CrossRef CAS PubMed.
- S. A. Krovi, M. M. Moreno Caffaro, S. Aravamudhan, N. P. Mortensen and L. M. Johnson, Fabrication of Nylon-6 and Nylon-11 Nanoplastics and Evaluation in Mammalian Cells, Nanomaterials, 2022, 12, 2699 CrossRef CAS PubMed.
- S. Huang, X. Huang, R. Bi, Q. Guo, X. Yu, Q. Zeng, Z. Huang, T. Liu, H. Wu, Y. Chen, J. Xu, Y. Wu and P. Guo, Detection and Analysis of Microplastics in Human Sputum, Environ. Sci. Technol., 2022, 56, 2476–2486 CrossRef CAS PubMed.
- Z. Li, Y. Gao, Q. Wu, B. Yan and X. Zhou, Quantifying the occurrence of polystyrene nanoplastics in environmental solid matrices via pyrolysis-gas chromatography/mass spectrometry, J. Hazard. Mater., 2022, 440, 129855 CrossRef CAS.
- H. Chen, X. Zhang, S. Xing, Z. Hao, B. Chen and Y.-G. Zhu, Quantifying Nanoplastics in Soil-Cultured Plants Based on a Microcombustion Calorimeter, Environ. Sci. Technol. Lett., 2023, 10, 1130–1134 CrossRef CAS.
- X.-X. Zhou, S. He, Y. Gao, Z.-C. Li, H.-Y. Chi, C.-J. Li, D.-J. Wang and B. Yan, Protein Corona-Mediated Extraction for Quantitative Analysis of Nanoplastics in Environmental Waters by Pyrolysis Gas Chromatography/Mass Spectrometry, Anal. Chem., 2021, 93, 6698–6705 CrossRef CAS PubMed.
- G. L. Sullivan, J. D. Gallardo, E. W. Jones, P. J. Hollliman, T. M. Watson and S. Sarp, Detection of trace sub-micron (nano) plastics in water samples using pyrolysis-gas chromatography time of flight mass spectrometry (PY-GCToF), Chemosphere, 2020, 249, 126179 CrossRef CAS PubMed.
- A.-K. Kniggendorf, C. Wetzel and B. Roth, Microplastics Detection in Streaming Tap Water with Raman Spectroscopy, Sensors, 2019, 19, 1839 CrossRef CAS PubMed.
- L. Zada, H. A. Leslie, A. D. Vethaak, G. H. Tinnevelt, J. J. Jansen, J. F. de Boer and F. Ariese, Fast microplastics identification with stimulated Raman scattering microscopy, J. Raman Spectrosc., 2018, 49, 1136–1144 CrossRef CAS.
- H. D. Frond, L. T. Hampton, S. Kotar, K. Gesulga, C. Matuch, W. Lao, S. B. Weisberg, C. S. Wong and C. M. Rochman, Monitoring microplastics in drinking water: An interlaboratory study to inform effective methods for quantifying and characterizing microplastics, Chemosphere, 2022, 298, 134282 CrossRef PubMed.
- F. Ribeiro, E. D. Okoffo, J. W. O'Brien, S. Fraissinet-Tachet, S. O'Brien, M. Gallen, S. Samanipour, S. Kaserzon, J. F. Mueller, T. Galloway and K. V. Thomas, Quantitative Analysis of Selected Plastics in High-Commercial-Value Australian Seafood by Pyrolysis Gas Chromatography Mass Spectrometry, Environ. Sci. Technol., 2020, 54, 9408–9417 CrossRef CAS PubMed.
- Y. Xu, Q. Ou, M. Jiao, G. Liu and J. P. van der Hoek, Identification and Quantification of Nanoplastics in Surface Water and Groundwater by Pyrolysis Gas Chromatography–Mass Spectrometry, Environ. Sci. Technol., 2022, 56, 4988–4997 Search PubMed.
- C. Way, M. D. Hudson, I. D. Williams, G. J. Langley and R. Marsh, Assessing the effectiveness of microplastic extraction methods on fishmeal with different properties, Anal. Methods, 2022, 14, 606–619 RSC.
- S.-Y. Lee, J. An and J.-H. Kwon, Sequential quantification of number and mass of microplastics in municipal wastewater using Fourier-transform infrared spectroscopy and pyrolysis gas chromatography-mass spectrometry, Environ. Pollut., 2023, 336, 122452 CrossRef CAS PubMed.
- M. S. M. Al-Azzawi, O. Knoop and J. E. Drewes, Validation of sample preparation methods for small microplastics (≤10 μm) in wastewater effluents, Chem. Eng. J., 2022, 446, 137082 CrossRef CAS.
- D. Kawecki and B. Nowack, Polymer-Specific Modeling of the Environmental Emissions of Seven Commodity Plastics As Macro- and Microplastics, Environ. Sci. Technol., 2019, 53, 9664–9676 Search PubMed.
- H. Deng, R. Wei, W. Luo, L. Hu, B. Li, Y. Di and H. Shi, Microplastic pollution in water and sediment in a textile industrial area, Environ. Pollut., 2020, 258, 113658 Search PubMed.
- B. R. Kiran, H. Kopperi and S. Venkata Mohan, Micro/nano-plastics occurrence, identification, risk analysis and mitigation: challenges and perspectives, Rev. Environ. Sci. Biotechnol., 2022, 21, 169–203 CrossRef PubMed.
- S. Mariano, S. Tacconi, M. Fidaleo, M. Rossi and L. Dini, Micro and Nanoplastics Identification: Classic Methods and Innovative Detection Techniques, Front. Toxicol., 2021, 3, 636640 CrossRef PubMed.
- C. Schür, S. Rist, A. Baun, P. Mayer, N. B. Hartmann and M. Wagner, When Fluorescence Is not a Particle: The Tissue Translocation of Microplastics in Daphnia magna Seems an Artifact, Environ. Toxicol. Chem., 2019, 38, 1495–1503 Search PubMed.
- V. K. Sharma, X. Ma, E. Lichtfouse and D. Robert, Nanoplastics are potentially more dangerous than microplastics, Environ. Chem. Lett., 2023, 21, 1933–1936 Search PubMed.
- G. V. Lowry, K. B. Gregory, S. C. Apte and J. R. Lead, Transformations of Nanomaterials in the Environment, Environ. Sci. Technol., 2012, 46, 6893–6899 CrossRef CAS PubMed.
- Y. K. Müller, T. Wernicke, M. Pittroff, C. S. Witzig, F. R. Storck, J. Klinger and N. Zumbülte, Microplastic analysis—are we measuring the same? Results on the first global comparative study for microplastic analysis in a water sample, Anal. Bioanal. Chem., 2020, 412, 555–560 CrossRef PubMed.
- A. Isobe, N. T. Buenaventura, S. Chastain, S. Chavanich, A. Cózar, M. DeLorenzo, P. Hagmann, H. Hinata, N. Kozlovskii, A. L. Lusher, E. Martí, Y. Michida, J. Mu, M. Ohno, G. Potter, P. S. Ross, N. Sagawa, W. J. Shim, Y. K. Song, H. Takada, T. Tokai, T. Torii, K. Uchida, K. Vassillenko, V. Viyakarn and W. Zhang, An interlaboratory comparison exercise for the determination of microplastics in standard sample bottles, Mar. Pollut. Bull., 2019, 146, 831–837 CrossRef CAS PubMed.
- C. Le Juge, B. Grassl, I. J. Allan and J. Gigault, Identification of polystyrene nanoplastics from natural organic matter in complex environmental matrices by pyrolysis–gas chromatography–mass spectrometry, Anal. Bioanal. Chem., 2023, 415, 2999–3006, DOI:10.1007/s00216-023-04609-7.
- S. Richter, J. Horstmann, K. Altmann, U. Braun and C. Hagendorf, A reference methodology for microplastic particle size distribution analysis: Sampling, filtration, and detection by optical microscopy and image processing, Appl. Res., 2023, 2, e202200055 Search PubMed.
- F. Caputo, R. Vogel, J. Savage, G. Vella, A. Law, G. Della Camera, G. Hannon, B. Peacock, D. Mehn, J. Ponti, O. Geiss, D. Aubert, A. Prina-Mello and L. Calzolai, Measuring particle size distribution and mass concentration of nanoplastics and microplastics: addressing some analytical challenges in the sub-micron size range, J. Colloid Interface Sci., 2021, 588, 401–417 CrossRef CAS PubMed.
- S. G. Bevers, C. Smith, S. Brown, N. Malone, D. H. Fairbrother, A. J. Goodman and J. F. Ranville, Improved methodology for the analysis of polydisperse engineered and natural colloids by single particle inductively coupled plasma mass spectrometry (spICP-MS), Environ. Sci.: Nano, 2023, 10, 3136–3148 RSC.
- F. Parrella, S. Brizzolara, M. Holzner and D. M. Mitrano, Impact of heteroaggregation between microplastics and algae on particle vertical transport, Nat. Water, 2024, 2, 541–552 CrossRef CAS PubMed.
- C. E. Enyoh, A. W. Verla, E. N. Verla, F. C. Ibe and C. E. Amaobi, Airborne microplastics: a review study on method for analysis, occurrence, movement and risks, Environ. Monit. Assess., 2019, 191, 668 CrossRef CAS PubMed.
- J. Hämer, L. Gutow, A. Köhler and R. Saborowski, Fate of Microplastics in the Marine Isopod Idotea emarginata, Environ. Sci. Technol., 2014, 48, 13451–13458 CrossRef PubMed.
- D.-K. Lee, S. Jeon, Y. Han, S.-H. Kim, S. Lee, I. J. Yu, K. S. Song, A. Kang, W. S. Yun, S.-M. Kang, Y. S. Huh and W.-S. Cho, Threshold Rigidity Values for the Asbestos-like Pathogenicity of High-Aspect-Ratio Carbon Nanotubes in a Mouse Pleural Inflammation Model, ACS Nano, 2018, 12, 10867–10879 CrossRef CAS PubMed.
- J. I. Kwak and Y.-J. An, Length- and polymer-dependent ecotoxicities of microfibers to the earthworm Eisenia andrei, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2022, 257, 109354 Search PubMed.
- S. Santini, E. De Beni, T. Martellini, C. Sarti, D. Randazzo, R. Ciraolo, C. Scopetani and A. Cincinelli, Occurrence of Natural and Synthetic Micro-Fibers in the Mediterranean Sea: A Review, Toxics, 2022, 10, 391 Search PubMed.
- Y. Huo, F. A. Dijkstra, M. Possell and B. Singh, in Advances in Agronomy, Elsevier, 2022, vol. 175, pp. 1–132 Search PubMed.
- J. I. Kwak, H. Liu, D. Wang, Y. H. Lee, J.-S. Lee and Y.-J. An, Critical review of environmental impacts of microfibers in different environmental matrices, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2022, 251, 109196 CAS.
- P. P. Prabhu, K. Pan and J. N. Krishnan, Microplastics: Global occurrence, impact, characteristics and sorting, Front. Mar. Sci., 2022, 9, 893641, DOI:10.3389/fmars.2022.893641.
- A. L. Lusher, N. A. Welden, P. Sobral and M. Cole, Sampling, isolating and identifying microplastics ingested by fish and invertebrates, Anal. Methods, 2017, 9, 1346–1360 Search PubMed.
- C. Wang, J. Zhao and B. Xing, Environmental source, fate, and toxicity of microplastics, J. Hazard. Mater., 2021, 407, 124357 CrossRef CAS PubMed.
- G. C. Thalhammer-Thurner and P. Debbage, Albumin-based nanoparticles: small, uniform and reproducible, Nanoscale Adv., 2023, 5, 503–512 RSC.
- T. Silva, L. R. Pokhrel, B. Dubey, T. M. Tolaymat, K. J. Maier and X. Liu, Particle size, surface charge and concentration dependent ecotoxicity of three organo-coated silver nanoparticles: Comparison between general linear model-predicted and observed toxicity, Sci. Total Environ., 2014, 468–469, 968–976 CrossRef CAS PubMed.
- M. Rosa, J. E. Ward, S. E. Shumway, G. H. Wikfors, E. Pales-Espinosa and B. Allam, Effects of particle surface properties on feeding selectivity in the eastern oyster Crassostrea virginica and the blue mussel Mytilus edulis, J. Exp. Mar. Biol. Ecol., 2013, 446, 320–327 CrossRef.
- A. W. Verla, C. E. Enyoh, E. N. Verla and K. O. Nwarnorh, Microplastic–toxic chemical interaction: a review study on quantified levels, mechanism and implication, SN Appl. Sci., 2019, 1, 1400 CrossRef.
- A. Pradel, C. Catrouillet and J. Gigault, The environmental fate of nanoplastics: What we know and what we need to know about aggregation, NanoImpact, 2023, 29, 100453 CrossRef CAS PubMed.
- L. Rowenczyk, A. Dazzi, A. Deniset-Besseau, V. Beltran, D. Goudounèche, P. Wong-Wah-Chung, O. Boyron, M. George, P. Fabre, C. Roux, A. F. Mingotaud and A. ter Halle, Microstructure Characterization of Oceanic Polyethylene Debris, Environ. Sci. Technol., 2020, 54, 4102–4109 CrossRef CAS PubMed.
- A. F. R. M. Ramsperger, J. Jasinski, M. Völkl, T. Witzmann, M. Meinhart, V. Jérôme, W. P. Kretschmer, R. Freitag, J. Senker, A. Fery, H. Kress, T. Scheibel and C. Laforsch, Supposedly identical microplastic particles substantially differ in their material properties influencing particle-cell interactions and cellular responses, J. Hazard. Mater., 2022, 425, 127961 CrossRef CAS PubMed.
- I. E. Napper, A. Bakir, S. J. Rowland and R. C. Thompson, Characterisation, quantity and sorptive properties of microplastics extracted from cosmetics, Mar. Pollut. Bull., 2015, 99, 178–185 CrossRef CAS PubMed.
- W. Mei, G. Chen, J. Bao, M. Song, Y. Li and C. Luo, Interactions between microplastics and organic compounds in aquatic environments: A mini review, Sci. Total Environ., 2020, 736, 139472 CrossRef CAS PubMed.
- F. Mendrik, R. Fernández, C. R. Hackney, C. Waller and D. R. Parsons, Non-buoyant microplastic settling velocity varies with biofilm growth and ambient water salinity, Commun. Earth Environ., 2023, 4, 1–9 CrossRef.
- A. Serrano-Lotina, R. Portela, P. Baeza, V. Alcolea-Rodriguez, M. Villarroel and P. Ávila, Zeta potential as a tool for functional materials development, Catal. Today, 2023, 423, 113862 CrossRef CAS.
- Y. Xiao and M. R. Wiesner, Characterization of surface hydrophobicity of engineered nanoparticles, J. Hazard. Mater., 2012, 215–216, 146–151 CrossRef CAS PubMed.
- A. Weber, A. Schwiebs, H. Solhaug, J. Stenvik, A. M. Nilsen, M. Wagner, B. Relja and H. H. Radeke, Nanoplastics affect the inflammatory cytokine release by primary human monocytes and dendritic cells, Environ. Int., 2022, 163, 107173 CrossRef CAS PubMed.
- M. Roursgaard, M. Hezareh Rothmann, J. Schulte, I. Karadimou, E. Marinelli and P. Møller, Genotoxicity of Particles From Grinded Plastic Items in Caco-2 and HepG2 Cells, Front. Public Health, 2022, 10, 906430 CrossRef PubMed.
- M. Lotfi Choobbari, J. Ferguson, N. Van den Brande, T. Smith, T. Chalyan, W. Meulebroeck and H. Ottevaere, Studying the concentration of polymers in blended microplastics using 2D and 3D Raman mapping, Sci. Rep., 2023, 13, 7771 CrossRef CAS PubMed.
- L. A. Utracki, P. Mukhopadhyay and R. K. Gupta, in Polymer Blends Handbook, ed. L. A. Utracki and C. A. Wilkie, Springer Netherlands, Dordrecht, 2014, pp. 3–170 Search PubMed.
- S. L. Wright and F. J. Kelly, Plastic and Human Health: A Micro Issue?, Environ. Sci. Technol., 2017, 51, 6634–6647 CrossRef CAS PubMed.
- C. Campanale, C. Massarelli, I. Savino, V. Locaputo and V. F. Uricchio, A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health, Int. J. Environ. Res. Public Health, 2020, 17, 1212 CrossRef CAS PubMed.
- J. N. Hahladakis, C. A. Velis, R. Weber, E. Iacovidou and P. Purnell, An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling, J. Hazard. Mater., 2018, 344, 179–199 CrossRef CAS PubMed.
- L. C. de Sá, L. G. Luís and L. Guilhermino, Effects of microplastics on juveniles of the common goby (Pomatoschistus microps): Confusion with prey, reduction of the predatory performance and efficiency, and possible influence of developmental conditions, Environ. Pollut., 2015, 196, 359–362 CrossRef PubMed.
- G. Jiménez-Skrzypek, C. Ortega-Zamora, J. González-Sálamo, C. Hernández-Sánchez and J. Hernández-Borges, The current role of chromatography in microplastic research: Plastics chemical characterization and sorption of contaminants, J. Chromatogr. Open, 2021, 1, 100001 CrossRef.
- J. H. Bridson, R. Abbel, D. A. Smith, G. L. Northcott and S. Gaw, Release of additives and non-intentionally added substances from microplastics under environmentally relevant conditions, Environ. Adv., 2023, 12, 100359 CrossRef CAS.
- Y. Tokiwa, B. P. Calabia, C. U. Ugwu and S. Aiba, Biodegradability of Plastics, Int. J. Mol. Sci., 2009, 10, 3722–3742 CrossRef CAS PubMed.
- J. R. White, Polymer ageing: physics, chemistry or engineering? Time to reflect, C. R. Chim., 2006, 9, 1396–1408 CrossRef CAS.
- L. Li, M. Li, H. Deng, L. Cai, H. Cai, B. Yan, J. Hu and H. Shi, A straightforward method for measuring the range of apparent density of microplastics, Sci. Total Environ., 2018, 639, 367–373 CrossRef CAS PubMed.
- E. Uurasjärvi, S. Hartikainen, O. Setälä, M. Lehtiniemi and A. Koistinen, Microplastic concentrations, size distribution, and polymer types in the surface waters of a northern European lake, Water Environ. Res., 2020, 92, 149–156 CrossRef PubMed.
- C. Dibke, M. Fischer and B. M. Scholz-Böttcher, Microplastic Mass Concentrations and Distribution in German Bight Waters by Pyrolysis–Gas Chromatography–Mass Spectrometry/Thermochemolysis Reveal Potential Impact of Marine Coatings: Do Ships Leave Skid Marks?, Environ. Sci. Technol., 2021, 55, 2285–2295, DOI:10.1021/acs.est.0c04522.
- Y. Xu, Q. Ou, X. Wang, F. Hou, P. Li, J. P. van der Hoek and G. Liu, Assessing the Mass Concentration of Microplastics and Nanoplastics in Wastewater Treatment Plants by Pyrolysis Gas Chromatography–Mass Spectrometry, Environ. Sci. Technol., 2023, 57, 3114–3123 Search PubMed.
- C. Goedecke, P. Eisentraut, K. Altmann, A. M. Elert, C. G. Bannick, M. Ricking, N. Obermaier, A.-K. Barthel, T. Schmitt, M. Jekel and U. Braun, Development of a Routine Screening Method for the Microplastic Mass Content in a Wastewater Treatment Plant Effluent, Front. Environ. Chem., 2022, 3, 844633, DOI:10.3389/fenvc.2022.844633.
- J. Hwang, D. Choi, S. Han, S. Y. Jung, J. Choi and J. Hong, Potential toxicity of polystyrene microplastic particles, Sci. Rep., 2020, 10, 7391 CrossRef CAS PubMed.
- E. J. Petersen, A. C. Barrios, T. B. Henry, M. E. Johnson, A. A. Koelmans, A. R. Montoro Bustos, J. Matheson, M. Roesslein, J. Zhao and B. Xing, Potential Artifacts and Control Experiments in Toxicity Tests of Nanoplastic and Microplastic Particles, Environ. Sci. Technol., 2022, 56, 15192–15206 Search PubMed.
- T. Yang and B. Nowack, A Meta-analysis of Ecotoxicological Hazard Data for Nanoplastics in Marine and Freshwater Systems, Environ. Toxicol. Chem., 2020, 39, 2588–2598 CrossRef CAS PubMed.
- T. A. Lewandowski, A. W. Hayes and B. D. Beck, Risk evaluation of occupational exposure to methylene dianiline and toluene diamine in polyurethane foam, Hum. Exp. Toxicol., 2005, 24, 655–662 CrossRef CAS PubMed.
- D. Lithner, Å. Larsson and G. Dave, Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition, Sci. Total Environ., 2011, 409, 3309–3324 CrossRef CAS PubMed.
- J. Ding, J. Li, C. Sun, F. Jiang, P. Ju, L. Qu, Y. Zheng and C. He, Detection of microplastics in local marine organisms using a multi-technology system, Anal. Methods, 2018, 11, 78–87 Search PubMed.
- T. Naidoo, Sershen, R. C. Thompson and A. Rajkaran, Quantification and characterisation of microplastics ingested by selected juvenile fish species associated with mangroves in KwaZulu-Natal, South Africa, Environ. Pollut., 2020, 257, 113635 Search PubMed.
- Y. Yao, M. Glamoclija, A. Murphy and Y. Gao, Characterization of microplastics in indoor and ambient air in northern New Jersey, Environ. Res., 2022, 207, 112142 Search PubMed.
- M. Pivokonsky, L. Cermakova, K. Novotna, P. Peer, T. Cajthaml and V. Janda, Occurrence of microplastics in raw and treated drinking water, Sci. Total Environ., 2018, 643, 1644–1651 CrossRef CAS PubMed.
- S. Monira, R. Roychand, M. A. Bhuiyan, F. I. Hai and B. K. Pramanik, Identification, classification and quantification of microplastics in road dust and stormwater, Chemosphere, 2022, 299, 134389 CrossRef CAS PubMed.
- S. Piehl, A. Leibner, M. G. J. Löder, R. Dris, C. Bogner and C. Laforsch, Identification and quantification of macro- and microplastics on an agricultural farmland, Sci. Rep., 2018, 8, 17950 Search PubMed.
- J. C. Aguilar-Guzmán, K. Bejtka, M. Fontana, E. Valsami-Jones, A. M. Villezcas, R. Vazquez-Duhalt and A. G. Rodríguez-Hernández, Polyethylene terephthalate nanoparticles effect on RAW 264.7 macrophage cells, Microplast. Nanoplast., 2022, 2, 9 CrossRef.
- L. Sun, S. Sun, M. Bai, Z. Wang, Y. Zhao, Q. Huang, C. Hu and X. Li, Internalization of polystyrene microplastics in Euglena gracilis and its effects on the protozoan photosynthesis and motility, Aquat. Toxicol., 2021, 236, 105840 CrossRef CAS PubMed.
- M. C. González-Caballero, M. de Alba González, M. Torres-Ruiz, P. Iglesias-Hernández, V. Zapata, M. C. Terrón, M. Sachse, M. Morales, R. Martin-Folgar, I. Liste and A. I. Cañas-Portilla, Internalization and toxicity of polystyrene nanoplastics on inmortalized human neural stem cells, Chemosphere, 2024, 355, 141815 CrossRef PubMed.
- F. Bertelà, C. Battocchio, G. Iucci, S. Ceschin, D. Di Lernia, F. Mariani, A. Di Giulio, M. Muzzi and I. Venditti, Dye-Doped Polymeric Microplastics: Light Tools for Bioimaging in Test Organisms, Polymers, 2023, 15, 3245 CrossRef PubMed.
- R. A. Murray, A. Escobar, N. G. Bastús, P. Andreozzi, V. Puntes and S. E. Moya, Fluorescently labelled nanomaterials in nanosafety research: Practical advice to avoid artefacts and trace unbound dye, NanoImpact, 2018, 9, 102–113 CrossRef.
- J. Kaur, E. Kelpsiene, G. Gupta, I. Dobryden, T. Cedervall and B. Fadeel, Label-free detection of polystyrene nanoparticles in Daphnia magna using Raman confocal mapping, Nanoscale Adv., 2023, 5, 3453–3462 RSC.
- M. Fischer and B. M. Scholz-Böttcher, Simultaneous Trace Identification and Quantification of Common Types of Microplastics in Environmental Samples by Pyrolysis-Gas Chromatography–Mass Spectrometry, Environ. Sci. Technol., 2017, 51, 5052–5060 CrossRef CAS PubMed.
- M. Dong, Z. She, X. Xiong, G. Ouyang and Z. Luo, Automated analysis of microplastics based on vibrational spectroscopy: are we measuring the same metrics?, Anal. Bioanal. Chem., 2022, 414, 3359–3372, DOI:10.1007/s00216-022-03951-6.
- E. Martínez-Francés, B. van Bavel, R. Hurley, L. Nizzetto, S. Pakhomova, N. T. Buenaventura, C. Singdahl-Larsen, M. L. T. Magni, J. E. Johansen and A. Lusher, Innovative reference materials for method validation in microplastic analysis including interlaboratory comparison exercises, Anal. Bioanal. Chem., 2023, 415, 2907–2919 CrossRef PubMed.
- Y. Huang, J. Chapman, Y. Deng and D. Cozzolino, Rapid measurement of microplastic contamination in chicken meat by mid infrared spectroscopy and chemometrics: A feasibility study, Food Control, 2020, 113, 107187 CrossRef CAS.
- G. L. Sullivan, J. D. Gallardo, E. W. Jones, P. J. Hollliman, T. M. Watson and S. Sarp, Detection of trace sub-micron (nano) plastics in water samples using pyrolysis-gas chromatography time of flight mass spectrometry (PY-GCToF), Chemosphere, 2020, 249, 126179 CrossRef CAS PubMed.
- T. Lauschke, G. Dierkes, P. Schweyen and T. A. Ternes, Evaluation of poly(styrene-d5) and poly(4-fluorostyrene) as internal standards for microplastics quantification by thermoanalytical methods, J. Anal. Appl. Pyrolysis, 2021, 159, 105310 CrossRef CAS.
- E. Miller, K. Yamahara, C. French, N. Spingarn, J. Birch and K. S. V. Houtan, A Raman spectral reference library of potential anthropogenic and biological ocean polymers, Sci. Data, 2021, 9, 780 CrossRef PubMed.
- I. Park, H. Kim and S. Lee, Characteristics of tire wear particles generated in a laboratory simulation of tire/road contact conditions, J. Aerosol Sci., 2018, 124, 30–40 Search PubMed.
- M. Kovochich, M. Liong, J. A. Parker, S. C. Oh, J. P. Lee, L. Xi, M. L. Kreider and K. M. Unice, Chemical mapping of tire and road wear particles for single particle analysis, Sci. Total Environ., 2021, 757, 144085 CrossRef CAS PubMed.
- S. Wagner, T. Hüffer, P. Klöckner, M. Wehrhahn, T. Hofmann and T. Reemtsma, Tire wear particles in the aquatic environment - A review on generation, analysis, occurrence, fate and effects, Water Res., 2018, 139, 83–100 CrossRef CAS PubMed.
- J. Brahney, N. Mahowald, M. Prank, G. Cornwell, Z. Klimont, H. Matsui and K. A. Prather, Constraining the atmospheric limb of the plastic cycle, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2020719118 Search PubMed.
- A. Giusti, R. Atluri, R. Tsekovska, A. Gajewicz, M. D. Apostolova, C. L. Battistelli, E. A. J. Bleeker, C. Bossa, J. Bouillard, M. Dusinska, P. Gómez-Fernández, R. Grafström, M. Gromelski, Y. Handzhiyski, N. R. Jacobsen, P. Jantunen, K. A. Jensen, A. Mech, J. M. Navas, P. Nymark, A. G. Oomen, T. Puzyn, K. Rasmussen, C. Riebeling, I. Rodriguez-Llopis, S. Sabella, J. R. Sintes, B. Suarez-Merino, S. Tanasescu, H. Wallin and A. Haase, Nanomaterial grouping: Existing approaches and future recommendations, NanoImpact, 2019, 16, 100182 Search PubMed.
- L. Lamon, D. Asturiol, A. Richarz, E. Joossens, R. Graepel, K. Aschberger and A. Worth, Grouping of nanomaterials to read-across hazard endpoints: from data collection to assessment of the grouping hypothesis by application of chemoinformatic techniques, Part. Fibre Toxicol., 2018, 15, 37 CrossRef CAS PubMed.
- N. K. Geitner, C. Ogilvie Hendren, G. Cornelis, R. Kaegi, J. R. Lead, G. V. Lowry, I. Lynch, B. Nowack, E. Petersen, E. Bernhardt, S. Brown, W. Chen, C. de Garidel-Thoron, J. Hanson, S. Harper, K. Jones, F. von der Kammer, A. Kennedy, J. Kidd, C. Matson, C. D. Metcalfe, J. Pedersen, W. J. G. M. Peijnenburg, J. T. K. Quik, S. M. Rodrigues, J. Rose, P. Sayre, M. Simonin, C. Svendsen, R. Tanguay, N. Tefenkji, T. van Teunenbroek, G. Thies, Y. Tian, J. Rice, A. Turner, J. Liu, J. Unrine, M. Vance, J. C. White and M. R. Wiesner, Harmonizing across environmental nanomaterial testing media for increased comparability of nanomaterial datasets, Environ. Sci.: Nano, 2020, 7, 13–36 RSC.
- K. Mills, M. L. Ostraat, K. Guzan and D. Murry, The Nanomaterial Registry: facilitating the sharing and analysis of data in the diverse nanomaterial community, Int. J. Nanomed., 2013, 7 CrossRef PubMed.
- X. Yan, A. Sedykh, W. Wang, B. Yan and H. Zhu, Construction of a web-based nanomaterial database by big data curation and modeling friendly nanostructure annotations, Nat. Commun., 2020, 11, 2519 CrossRef CAS PubMed.
- ISO/DIS 16094-2, https://www.iso.org/standard/84460.html, (accessed 10 February 2025).
- ISO/DIS 16094-3, https://www.iso.org/standard/84463.html, (accessed 10 February 2025).
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4en00767k |
| ‡ Contributed equally to this work. |
| § https://inframes.pratt.duke.edu/about. |
| This journal is © The Royal Society of Chemistry 2025 |