Exploring new frontiers: alliance of pyrazole and thiadiazole in energetic materials†
Parasar
Kumar
 a,
Vikas D.
Ghule
a,
Vikas D.
Ghule
 b and
Srinivas
Dharavath
b and
Srinivas
Dharavath
 *a
*a
aEnergetic Materials Laboratory, Department of Chemistry, Indian Institute of Technology Kanpur, Kanpur-208016, Uttar Pradesh, India. E-mail: srinivasd@iitk.ac.in
bDepartment of Chemistry, National Institute of Technology Kurukshetra, Kurukshetra-136119, Haryana, India
First published on 11th April 2025
Abstract
A series of high-performing C–C bonded nitropyrazole and thiadiazole-based energetic materials (compounds 3–7) were synthesized and thoroughly characterized. SC-XRD studies supported the structure of compounds 3, 4, 6, and 7. The synthesized compounds exhibited high densities (≥1.77 g cm−3), compounds 3 and 5 demonstrating admirable detonation properties (VOD = 8300 and 7265 m s−1; DP = 30.31 and 21.25 GPa, respectively), surpassing the present benchmark explosives HNS and TNT and setting new standards for sulfur-based energetic materials. Notably, compound 3 showed an ignition delay of 13 ms in a hot needle test, indicating its potential as an igniter.
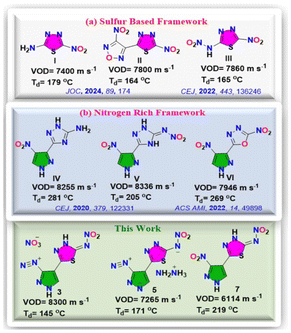
The design and development of new energetic materials represent a critical focus in materials science research.1,2 This field has been an active area of investigation due to the extensive applications of energetic materials in industries such as mining, construction, propellants, pyrotechnics, defense, aerospace, etc.3,4 The next generation of energetic materials aims to prioritize combined properties such as improved performance and safety, efficient-straightforward synthesis, environmental friendliness, and low toxicity.5,6 However, striking a balance between high detonation performance and low sensitivity is a significant challenge, as improved properties often come at the cost of molecular stability.7,8 Consequently, advancements in the next generation of high-energy-density materials (HEDMs) are not limited to innovative molecular designs but also integrate chemistry, crystallography, and physics to develop advanced energy sources.9,10 The field of energetic materials science has become remarkably diverse, with modern types of propellants, explosives, and pyrotechnics continually being developed for a wide range of applications.11,12 The newly synthesized or existing high-energy material must be evaluated based on specific functional properties, including detonation performance, mechanical sensitivity, oxygen balance, and thermal stability.13 The rational design of energetic molecular frameworks, combined with the selection and strategic arrangement of suitable functional groups, is supposed to be a key approach for developing molecular structures that achieve a balance between high energy and stability, addressing the dual demands of energy and safety in energetic materials.14,15 These high-energy materials are further classified according to their potential applications based on the above factors. Nitrogen-rich heterocyclic compounds are important backbones in energetic materials, with many high-performance energetic compounds incorporating at least one heterocyclic component in their structures.16 Energetic compounds containing carbon, hydrogen, nitrogen, and oxygen are the subject of extensive basic and applied research by scientific and military institutions globally.17,18 Notably, the redox process in the decomposition reaction that releases energy is solely driven by the oxygen atoms.19,20 Despite black powder being the oldest known explosive containing sulfur, research on sulfur-based energetic materials has been relatively scarce, resulting in a limited understanding of their decomposition and combustion mechanisms.21,22 The incorporation of explosophoric groups like nitro, azido, hydrazino, nitramino, dinitromethyl, and trinitromethyl into sulfur-containing nitrogen-rich HEDMs remains largely unexplored. The inclusion of sulfur into CHNO-based explosives can be highly beneficial, significantly improving their heat and chemical resistance, alignment with other propellant or explosive ingredients and overall safety characteristics.23 Lately, Gozin and colleagues presented a sulfur-containing nitramino-1,3,4-thiadiazole scaffold and explored the potential laser-ignition capabilities of sulfur-based energetic materials, highlighting their properties.24 Later, Fershtat and coworkers reported C–C linked 1,3,4-thiadiazole and furazan rings adorned with energetic –NO2 and –N
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– functionalities (Fig. 1a).25 Similar C–C bonded nitro pyrazoles with substituted triazoles and oxadiazoles have been reported earlier, which possess energetic properties comparable with sulfur-based energetic materials reported in this work (Fig. 1b).26,27 Keeping all this in mind, here we have synthesized C–C bonded substituted pyrazole and thiadiazole-based energetic materials from commercially available inexpensive starting materials in a straightforward way with complete spectroscopic and structural characterization. Caution! All the compounds investigated are potentially explosive, energetic materials. All manipulations must be carried out by using appropriate standard safety precautions.
N– functionalities (Fig. 1a).25 Similar C–C bonded nitro pyrazoles with substituted triazoles and oxadiazoles have been reported earlier, which possess energetic properties comparable with sulfur-based energetic materials reported in this work (Fig. 1b).26,27 Keeping all this in mind, here we have synthesized C–C bonded substituted pyrazole and thiadiazole-based energetic materials from commercially available inexpensive starting materials in a straightforward way with complete spectroscopic and structural characterization. Caution! All the compounds investigated are potentially explosive, energetic materials. All manipulations must be carried out by using appropriate standard safety precautions.
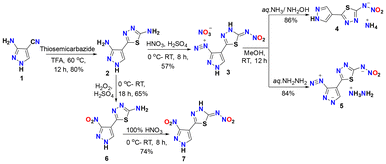
Compound 5-(3-amino-1H-pyrazol-4-yl)-1,3,4-thiadiazol-2-amine (2) was synthesized using 3-amino-1H-pyrazole-4-carbonitrile (1) and thiosemicabazide in trifluoroacetic acid (TFA) at 60 °C for 12 hours in 80% yield. Further, when compound 2 was treated with 100% nitric and conc. sulfuric acid, (Z)-4-(5-(nitroimino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)-1H-pyrazole-3-diazonium nitrate (3) was isolated in good yield (57%). Compound 3 was reacted with aqueous ammonia at room temperature led to the formation of ammonium (5-(1H-pyrazol-4-yl)-1,3,4-thiadiazol-2-yl)(nitro)amide (4) with the removal of diazonium group while the reaction with hydrazine hydrate forms hydrazinium 4-(5-(nitroamide)-1,3,4-thiadiazol-2-yl)-pyrazole-3-diazonium (5) in quantitative yields. Later, compound 2 was oxidized using a mixture of hydrogen peroxide and sulfuric acid to get 5-(3-nitro-1H-pyrazol-4-yl)-1,3,4-thiadiazol-2-amine (6) in 65% yield. Furthermore, compound 6 was nitrated using 100% nitric acid to get (Z)-N-(5-(3-nitro-1H-pyrazol-4-yl)-1,3,4-thiadiazol-2(3H)-ylidene)nitramide (7) in 74% yield as shown in Scheme 1. For the comprehensive analysis of the structural features, single crystals of compound 3 were obtained by quenching the reaction mixture in crushed ice, refrigerating it for 15 minutes, and subsequently placed at room temperature for overnight. It crystallized in monoclinic space group P21/n, having four molecules per unit cell. The two rings are tilted slightly with an angle of 13.36°. The presence of hydrogen bonding and non-covalent interaction assists the crystal to form wave-like crystal packing with the interlayer separation of 3.165 Å, as shown in Fig. 2.
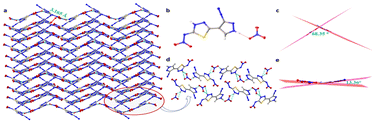 | ||
| Fig. 2 (a) Packing pattern along a-axis, (b) crystal structure, (c) angle between the two molecules of compound 3. | ||
In order to evaluate the structural integrity of compound 4, suitable crystals were obtained in methanol and water at ambient conditions using a slow evaporation technique. The compound crystallizes in triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , having two molecules per unit cell. The crystal arrangement forms the layer-by-layer packing with the interlayer separation of 3.288 Å. The crystal structure also exhibits hydrogen bonding networks and non-covalent interactions, which helps in stabilizing the crystal framework as shown in Fig. 3. Moreover, the layer-by-layer compact stacking arrangement demonstrates reduced sensitivity compared to alternative stacking configurations, as this packing pattern facilitates the conversion of mechanical energy applied to the bulk material into relative interlayer motion, particularly when subjected to significant external disturbances. To gain more insights into the structural characteristics of compound 7, suitable crystals were obtained through slow evaporation of dimethyl sulfoxide at ambient temperature.
, having two molecules per unit cell. The crystal arrangement forms the layer-by-layer packing with the interlayer separation of 3.288 Å. The crystal structure also exhibits hydrogen bonding networks and non-covalent interactions, which helps in stabilizing the crystal framework as shown in Fig. 3. Moreover, the layer-by-layer compact stacking arrangement demonstrates reduced sensitivity compared to alternative stacking configurations, as this packing pattern facilitates the conversion of mechanical energy applied to the bulk material into relative interlayer motion, particularly when subjected to significant external disturbances. To gain more insights into the structural characteristics of compound 7, suitable crystals were obtained through slow evaporation of dimethyl sulfoxide at ambient temperature.
 | ||
| Fig. 3 (a) Packing pattern along a-axis, (b) crystal structure, (c) interlayer separation, (d) H-bonding interaction along b-axis of compound 4. | ||
The compound crystallizes in monoclinic space group P21/c with four molecules per unit cell. The molecule shows lower crystal density due to DMSO presence. The crystal arrangement forms the wave-like packing with an interlayer separation of 3.241 Å. The hydrogen bonding and non-covalent interactions help in stabilizing the crystal framework as shown in Fig. 4. The physicochemical properties of all the synthesized compounds are listed in Table 1. The thermal stability/decomposition temperatures (Td) of the newly synthesized compounds were measured using TGA-DSC (SDT650) in an inert nitrogen atmosphere at the ramp rate of 5 °C min−1. The onset thermal decomposition of compounds 3–7 falls in the range of 145–219 °C. The diazonium salt 3 has a decomposition temperature of 145 °C, while compounds 4, 5, and 7 have a very good decomposition temperature of 219, 171, and 219 °C, respectively. The low decomposition temperature of compounds 3 and 5 may be due to the dual presence of sensitive explosophoric groups such as nitrimino and diazonium in a single molecule. The thermal decomposition of compounds 4, 5, and 7 is greater than the reported sulfur-based compound 3-nitro-4-(5-nitro-1,3,4-thiadiazol-2-yl)-1,2,5-oxadiazole (II), which is 164 °C while 4 and 7 has Td even greater than RDX (204 °C). The room temperature densities of all the compounds were measured using a gas pycnometer in an inert helium environment. The densities (ρ) of all the compounds ranging from 1.61 to 1.84 g cm−3. Among all, compound 3 has a high density, which is 1.84 g cm−3, while 4, 5, and 7 have a density of 1.61, 1.77, and 1.79 g cm−3. The densities of compounds 4, 5, and 7 are greater than the TNT (1.65 g cm−3) and HNS (1.75 g cm−3). All geometry optimizations, vibrational frequency and potential energy calculations were carried out using the Gaussian 09 program with the M06-2X/def2-TZVPP method.28 The gas phase heats of formation (HOF) were computed by using isodesmic reaction approach. The solid-state HOF for compound 7 was estimated by subtracting the sublimation enthalpy from the gas-phase HOF. Jenkins method based on the Born–Haber cycle was applied to obtain the HOF of energetic salts 3–5.29 The detailed description of these methods is given in the ESI.† All the compounds show positive heats of formation ranging from 369.2 to 811.3 kJ mol−1, which are better than those of TNT (−59.3 kJ mol−1), HNS (78.0 kJ mol−1), and RDX (70.0 kJ mol−1). The HOF of compounds 3 (678.5 kJ mol−1) and 5 (811.3 kJ mol−1) is even greater than compound II (554.0 kJ mol−1). The detonation parameters, such as detonation velocity (VOD) and detonation pressure (DP) for all synthesized compounds, were assessed by Explo-5 (v7.01.01) code using the measured densities and calculated HOFs.30 Among all, compounds 3 and 5 show the highest VODs of 8300 and 7265 m s−1 and DPs of 30.13 and 21.25 GPa, which is greater than TNT (VOD = 6820 m s−1, DP = 19.5 GPa), HNS (VOD = 7164 m s−1, DP = 21.65 GPa) while compound 3 has even higher than previously reported SEMol 3-nitro-4-(5-nitro-1,3,4-thiadiazol-2-yl)-1,2,5-oxadiazole II (VOD = 7800 m s−1, DP = 27.0 GPa). It is worth mentioning that, to the best of our knowledge, compound 3 has the highest properties among all sulfur-based compounds reported so far in the literature.
| Comp |
T
d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a [°C] a [°C] |
ρ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b [g cm−3] b [g cm−3] |
HOFc [kJ mol−1] | VODd [m s−1] | DPe [GPa] | ISf [J] | FSg [N] |
|---|---|---|---|---|---|---|---|
a Onset thermal decomposition temperature under nitrogen environment at 5 °C min−1.
b Gas pycnometer density at RT.
c Computed heat of formation (solid).
d Detonation velocity.
e Detonation pressure.
f Impact sensitivity.
g Friction sensitivity.
h ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ref. 25.
i Ref. 25.
i ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ref. 2.
j Ref. 2.
j ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ref. 10.
k Ref. 10.
k ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ref. 2.
l Ref. 2.
l ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ref. 15. Ref. 15.
|
|||||||
| 3 | 145 | 1.84 | 678.5 | 8300 | 30.31 | 2.5 | 60 |
| 4 | 219 | 1.61 | 387.6 | 6391 | 16.02 | 40 | 360 |
| 5 | 171 | 1.77 | 811.3 | 7265 | 21.25 | 40 | 360 |
| 7 | 219 | 1.79 | 369.2 | 6114 | 16.02 | 30 | 360 |
| II | 164 | 1.75 | 554.0 | 7800 | 27.00 | 5 | 160 |
| TNT | 300 | 1.65 | −59.3 | 6820 | 19.50 | 39.2l | 353 |
| HNS | 318 | 1.75 | 78.0 | 7164 | 21.65 | 11.5l | 240 |
| RDX | 204 | 1.82 | 70.7 | 8748 | 34.90 | 7.5 | 120 |
Ensuring the safe nature of materials to accidental detonation and mechanical stimuli while handling, storage, and transport has been a top priority in this field, and for that, impact sensitivity (IS) and friction sensitivity (FS) tests were conducted using a BAM fall hammer and a friction tester. All the synthesized compounds were assessed for their mechanical sensitivity in powdered form. Compound 3 was observed to be sensitive towards impact (2.5 J) and friction (60 N), but all remaining compounds are insensitive to impact sensitivity (>30 J) and friction sensitivity (>360 N).
A hot needle test for compound 3 was also carried out, which revealed its potential nature as an igniter. Interestingly, compound 3 exhibits rapid ignition delay time, which begins at 13 ms, and the flame vanished at 326 ms, having a faint luminous flame (Fig. S30†).
In summary, this study highlights the successful synthesis of carbon–carbon bonded nitro pyrazole and thiadiazole-based sulfur-containing energetic materials (compounds 3–7), showcasing their admirable properties. Among these, compounds 3 and 5 stand out with exceptional detonation velocities of 8300 and 7265 m s−1 and detonation pressures of 30.31 and 21.25 GPa, respectively, surpassing the performance of many benchmark explosives such as HNS and TNT. Notably, compound 3, with its unique diazonium salt structure, demonstrated an ignition delay of just 13 ms in a hot needle test, making it suitable for use as an igniter. Compounds 4 and 7 exhibited impressive thermal stability with a decomposition temperature of 219 °C, further broadening their potential applications. Compounds 3 and 5 are among the most advanced sulfur-based nitrogen-rich energetic materials reported to date, representing a significant advancement in the field of energetic materials.
Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
PK thanks IIT Kanpur for the research fellowship and infrastructure. SD is grateful for the financial support from the core research grant (ANRF-CRG/2023/000007), The Anusandhan National Research Foundation-Science and Engineering Research Board, Department of Science and Technology, Government of India.References
- P. Yin, Q. Zhang and J. M. Shreeve, Acc. Chem. Res., 2016, 49, 4–16 CrossRef CAS PubMed.
- T. M. Klapötke, Chemistry of High-Energy Materials, Walter de Gruyter GmbH & Co KG, 2022 Search PubMed.
- Z. Yin, W. Huang, Z. Zeng, Y. Liu, J. M. Shreeve and Y. Tang, ACS Appl. Mater. Interfaces, 2022, 14, 49847–49853 CrossRef CAS PubMed.
- N. V. Muravyev, L. Fershtat and Q. Zhang, Chem. Eng. J., 2024, 486, 150410 CrossRef CAS.
- S. Banik, P. Kumar, V. D. Ghule, S. Khanna, D. Allimuthu and S. Dharavath, J. Mater. Chem. A, 2022, 10, 22803–22811 RSC.
- M. Benz, T. M. Klapotke, J. Stierstorfer and M. Voggenreiter, J. Am. Chem. Soc., 2022, 144, 6143–6147 CrossRef CAS PubMed.
- S. R. Yocca, M. Zeller, E. F. C. Byrd and D. G. Piercey, J. Mater. Chem. A, 2022, 10, 1876–1884 RSC.
- O. T. O'Sullivan and M. J. Zdilla, Chem. Rev., 2020, 120, 5682–5744 CrossRef PubMed.
- H. Gao and J. M. Shreeve, Chem. Rev., 2011, 111, 7377–7436 CrossRef CAS PubMed.
- R. Rajak, P. Kumar, V. D. Ghule and S. Dharavath, Inorg. Chem., 2023, 62, 8389–8396 CrossRef CAS PubMed.
- J. Li, Y. Liu, W. Ma, T. Fei, C. He and S. Pang, Nat. Commun., 2022, 13, 5697 Search PubMed.
- A. Abdelaziz, D. Trache, A. F. Tarchoun, H. Boukeciat, D. E. Kadri, H. Hassam, S. Ouahioune, N. Sahnoun, S. Thakur and T. M. Klapötke, Chem. Eng. J., 2024, 487, 150654 CrossRef CAS.
- P. Kumar, V. D. Ghule and S. Dharavath, Mater. Adv., 2024, 5, 6399–6404 RSC.
- N. Makhova, L. Belen, G. Gazieva, I. Dalinger, L. Konstantinova, V. Kuznetsov, A. Kravchenko, M. Krayushkin, O. Rakitin, A. Starosotnikov, L. Fershtat, S. Shevelev, V. Shirinian and V. Yarovenko, Russ. Chem. Rev., 2020, 89, 55–124 Search PubMed.
- (a) S. Zeman and V. B. Patil, FirePhysChem, 2025, 5, 68–73 CrossRef; (b) M. Jujam, R. Rajak and S. Dharavath, Adv. Funct. Mater., 2025, 35, 2412638 CrossRef CAS.
- G. Steinhauser and T. M. Klapötke, Angew. Chem., Int. Ed., 2008, 47, 3330–3347 CrossRef CAS PubMed.
- Q. Lai, Y. Long, P. Yin, J. M. Shreeve and S. Pang, Acc. Chem. Res., 2024, 57, 2790–2803 CrossRef CAS PubMed.
- J. Yount and D. G. Piercey, Chem. Rev., 2022, 122, 8809–8840 Search PubMed.
- Z. Xu, G. Cheng, S. Zhu, Q. Lin and H. Yang, J. Mater. Chem. A, 2018, 6, 2239–2248 RSC.
- Y. Tang, C. He, L. A. Mitchell, D. A. Parrish and J. M. Shreeve, Angew. Chem., Int. Ed., 2016, 55, 5565–5567 CrossRef CAS PubMed.
- F. Seel, Stud. Inorg. Chem., 1984, 5, 55–66 CAS.
- E. C. Koch and M. Sućeska, Z. Anorg. Allg. Chem., 2021, 647, 192–199 CrossRef CAS.
- P. Kumar, V. D. Ghule and S. Dharavath, Dalton Trans., 2024, 53, 19112–19115 RSC.
- J. Das, D. Shem-Tov, S. Zhang, C. Z. Gao, L. Zhang, C. Yao, E. Flaxer, J. Stierstorfer, M. Wurzenberger, I. Rahinov and M. Gozin, Chem. Eng. J., 2022, 443, 136246 CrossRef CAS.
- I. D. Deltsov, I. V. Ananyev, D. B. Meerov and L. L. Fershtat, J. Org. Chem., 2024, 89, 174–182 CrossRef CAS PubMed.
- Q. Ma, G. Zhang, J. Li, Z. Zhang, H. Lu, L. Liao, G. Fan and F. Nie, Chem. Eng. J., 2020, 379, 122331 CrossRef CAS.
- A. K. Yadav, V. D. Ghule and S. Dharavath, ACS Appl. Mater. Interfaces, 2022, 14, 49898–49908 CrossRef CAS PubMed.
- M. J. Frisch, et al., Gaussian, Inc., Wallingford CT, 2013.
- H. D. B. Jenkins, D. Tudela and L. Glasser, Inorg. Chem., 2002, 41, 2364–2367 CrossRef CAS PubMed.
- M. Sućeska, EXPLO5, version 7.01.01, Brodarski Institute, Zagreb, Croatia, 2013 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2419623–2419626. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5dt00730e |
| This journal is © The Royal Society of Chemistry 2025 |