Modulation of phosphor luminescence performance by high concentration self-sensitization of Er and Ho–Yb ion co-doping under 1550 nm excitation
Bohan
Lei
,
Liping
Lu
 * and
Haiying
Sun
* and
Haiying
Sun
School of Materials Science and Engineering, Changchun University of Science and Technology, Changchun 130022, China. E-mail: luliping771219@126.com
First published on 30th May 2024
Abstract
In this paper, upconversion fluoride phosphors NaY1−x−y−zF4:Erx3+, Hoy3+, Ybz3+ were synthesized by the low-temperature combustion method. And the optimal ratio of lanthanide ion doping in the matrix lattice was determined by the control variable method. First of all, the experimental results show that some Er3+-2 ions may be present in the samples doped with a high concentration of Er3+ ions, and the energy carried by the 1550 nm photons is absorbed by them and transferred to the remaining Er3+-1 ions in the form of sensitizers, which leads to saturation excitation of these Er3+-1 ions. Thus, the upconversion luminescence intensity of the Er3+ ion-doped samples was dramatically increased. Secondly, it was found that the singly doped Ho3+ ion samples also showed significant absorption of 1550 nm photons. In contrast, doping Yb3+ ions in samples singly doped with Ho3+ ions produces the opposite effect of Er3+ ions. The upconversion luminescence intensity of the Ho3+ ion-doped samples is significantly quenched. According to the above experimental phenomena, when a small amount of Ho3+ ions are doped into the matrix lattice of the sample doped with a high concentration of Er3+ ions, firstly, these Ho3+ ions can act as transient energy transition centers in the lattice. Secondly, they can also play the role of another self-absorption activation center in the matrix lattice. The upconversion luminescence performance of the Er3+–Ho3+ ion co-doped samples is significantly enhanced, so the characteristic emissions of Er3+ and Ho3+ ions are highly overlapped in the visible region. A small amount of Yb3+ ions continue to be doped into the Er3+–Ho3+ ion co-doped system, due to the significant quenching effect of the Yb3+ ions on the luminescence of the Ho3+ ions. The Yb3+ ions mainly play the role of reverse energy transfer centers between the Er3+–Yb3+ ions in the crystal lattice. This results in the upconversion luminescence intensity of the triple-doped samples being enhanced significantly by increasing the utilization of the system for the 1550 nm photons. In this paper, the phase composition and morphology of the phosphors were studied by an X-ray diffractometer and scanning electron microscope. The upconversion luminescence mechanism of Er3+–Ho3+–Yb3+ ion triple-doped samples under 1550 nm excitation and the sensitization interactions between the ions were systematically investigated by upconversion emission spectra and fluorescence lifetime. This work provides a new idea for the design of high-color purity upconversion luminescent phosphors under 1550 nm excitation, and the prepared phosphors can be applied in the field of display lighting.
1. Introduction
Upconversion luminescence is an anti-Stokes process by which low-energy infrared light can be converted into high-energy visible light.1 Therefore, it has been widely studied in infrared detection, optical anti-counterfeiting, and bio-detection.2–4 Currently, the research on upconversion luminescence is mainly concentrated in the 980 nm band,5 but the research on the 1550 nm band is relatively rare. This is mainly due to the lack of effective sensitizer ions in the 1550 nm band.6 However, compared with the 980 nm band, the 1550 nm band has strong anti-interference ability, strong anti-light scattering ability, and safety for living organisms.7 Hence, the 1550 nm band has better application prospects. Among lanthanide ions, Er3+ ions have the largest absorption cross-section for 1550 nm photons, so Er3+ ions have potential as sensitizer ions in this band.6 However, if only relying on the doping of Er3+ ions to achieve a good response of the matrix lattice to 1550 nm photons, it will inevitably lead to an increase in the doping concentration of Er3+ ions.8 In addition, the upconversion luminescence intensity of the samples under 1550 nm excitation is also limited by the maximum doping concentration of activator ions in the matrix lattice. Hence, the upconversion luminescence of samples in this band is generally weak. Therefore, it is crucial that the cross-relaxation and concentration quenching chances induced by high-concentration doping of Er3+ ions are attenuated. In this paper, it is found that high-concentration doping of Er3+ ions can be realized in the NaYF4 matrix lattice. It is also found that at low-concentration doping, Er3+ ions mainly play the role of unsaturated luminescent centers in the lattice. As the doping concentration of Er3+ ions continued to increase, firstly, some of the Er3+-2 ions can act as sensitizers to absorb and transfer energy. This causes the remaining Er3+-1 ions to be saturated with excitation so that the upconversion luminescence of the sample is significantly enhanced. Secondly, 1550 nm photons can be well responded by the Ho3+ ions in lanthanide activator ions, so mutual sensitization between the Er3+ and Ho3+ ions can be present. Since the characteristic emissions of Er3+ and Ho3+ ions are highly overlapping in the visible region, the upconversion luminescence intensity of the sample is significantly enhanced by the additive effect of the two characteristic emissions. At this time, Ho3+ ions mainly play the roles of transient energy trapping centers and new luminescence centers in the matrix lattice. On this basis, we also find that the upconversion luminescence intensity of the samples is further enhanced by doping a small amount of Yb3+ ions into the above two co-doping systems. At this time, the Yb3+ ions mainly play the role of the reverse energy transfer center in the matrix lattice. The energy that should have been dissipated in the supersaturated excitation is stored in the excited state energy level of the Yb3+ ions through the energy transfer process. Then, the Er3+ ions are secondarily excited by the reverse energy transfer process. Due to this process, the utilization of photons by the sample is drastically increased. As a result, the upconversion luminescence intensity of the triple-doped system is significantly enhanced.In this paper, upconversion fluoride phosphors NaY1−x−y−zF4:Erx3+, Hoy3+, Ybz3+ were synthesized by the low temperature combustion method. The inter-sensitization interaction between Er3+–Ho3+–Yb3+ ions was investigated in detail. This study not only provides a new idea for the design of upconversion luminescent materials, but also further improves the energy transfer mechanism between the upconversion multi-doped lanthanide activator and sensitizer ions under 1550 nm excitation.
2. Experimental
2.1 Preparation
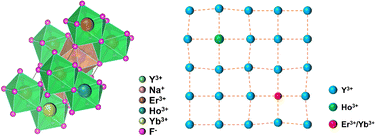
The experiments started with sodium fluoride (NaF), rare earth oxides Ln2O3 (Ln = Er, Y, Ho and Yb) as raw materials, ammonium hydrogen fluoride (NH4HF2), urea [CO(NH2)2], and ammonium nitrate (NH4NO3). The up-conversion fluoride phosphors NaY1−x−y−zF4:Erx3+, Hoy3+, Ybz3+ were synthesized by the low-temperature synthesized combustion (LCS) method. First, the rare earth oxides Ln2O3 were dissolved in dilute nitric acid to make a solution of rare earth nitrates [Ln(NO3)3]. The co-precipitation method was adopted to convert rare-earth nitrates into solid rare-earth fluorides (LnF3). Then the prepared fluoride raw materials were mixed with oxidizer [NH4NO3], reductant [CO(NH2)2] and NH4HF2 by adding alcohol to grind them well to obtain prefabricated combustion rods. Finally, they were ignited in a muffle furnace at 650 °C. 10 min later, the crucible was removed, and a fluffy pink powder was obtained. After the samples were cooled, they were gently ground and used for subsequent tests.2.2 Measurements
The X-ray diffraction (XRD) patterns of the samples were tested with a Rigaku/Ultima IV X-ray diffractometer at 40 kV, 20 mA, with Cu K radiation (λ = 1.5405 Å) and a diffraction angle range of 10 to 80 (2θ). The morphology of the samples was tested using a JSM-6701F scanning electron microscope (SEM) at an accelerating voltage of 10 kV. The upconversion emission spectra of the samples were tested by a Shimadzu RF-5301PC spectrometer and a power-tunable 1550 nm laser at room temperature, the spectrometer's test slit was 1.5 μm and the excitation power was 1.57 mW cm−2.3. Results and discussion
3.1 Crystal structure analysis
Fig. 1 and 2 show the XRD patterns of the samples doped with different kinds of lanthanides, respectively. Table 1 shows the ionic radius of the lanthanide ions doped in this sample. It is well known that when other lanthanide ions are doped in the matrix lattice, the difference in ion radius will cause the contraction or expansion of the cell of the matrix lattice, thus influencing the sample's up-conversion luminescence characteristics.9,10 The variation law of the matrix lattice can be described by the interplanar spacing of the hexagonal crystal system and Bragg's law.11,12 | (1) |
2d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = nλ θ = nλ | (2) |
| Elements | Y | Er | Ho | Yb |
|---|---|---|---|---|
| Ionic size [Å] | 0.9 | 0.89 | 0.901 | 0.868 |
 | ||
| Fig. 4 SEM images of the matrix lattice doped with different kinds of ions: (a) doped Er3+ ions, (b) doped Ho3+ ions and (c) doped Yb3+ ions. | ||
3.2 Modulating effect of the Er3+ ion dopant on the upconversion luminescence properties
Er3+ ions are often used as activator ions in the 1550 nm band due to their significant absorption of 1550 nm photons.17,18 Still, they are limited by the cross-relaxation between ions when doped with a high concentration of Er3+ ions.16,19 This has led to the fact that doping with high concentrations of Er3+ ions at 1550 nm excitation has been poorly studied.19 Therefore, the mechanism of the role played by Er3+ ions in the upconversion luminescence on the lattice at high doping concentrations is imperfectly studied. Hence, it is necessary to systematically study the mechanism of upconversion luminescence on Er3+ ions doped with high concentration.In this paper, NaYF4:Er3+ upconversion fluoride phosphors with different Er3+ ion doping concentrations were synthesized by the LCS method. The upconversion emission spectra were determined for different Er3+ ion doping concentrations, as shown in Fig. 5 and 6, and a schematic diagram of the energy transfer between Er3+ ion single-doped sample ions is also shown. As can be seen from the figure, the doping concentration of Er3+ ions gradually increases. The upconversion luminescence intensity of the sample increased first, then decreased, and then increased and then decreased. And thus, two inflection points of high and low upconversion luminescence intensity are generated, which correspond to the doping concentration of Er3+ ions of 12 mol% and 26 mol% respectively. The upconversion emission spectrum of the sample consists of 521 nm for green light, 544 nm for red light and 660 nm for red light. For the green emission of the sample, it corresponds to the 4I15/2 → 2H11/2 and 4I15/2 → 4S3/2 energy level transitions of Er3+ ions, and for the red emission of the sample, it corresponds to the 4I15/2 → 4F9/2 energy level transitions of Er3+ ions. For the red emission of the sample, it corresponds to the energy level transitions of the 4I15/2 → 4F9/2 energy levels of Er3+ ions. For Er3+ ions doped with a high concentration of 26 mol% and low concentration of 12 mol%, the total luminous intensity is increased by 1.62 times, and the red-green ratio is increased by 2.31 times. This is mainly due to the strong response of Er3+ ions to 1550 nm photons.6 The Er3+-1 ions are uniformly distributed in the NaYF4 matrix lattice for the inflection point appearing at low-concentration doping. The probability of the ions undergoing cross-relaxation is small because of the long energy transfer distance between neighboring Er3+ ions.20 In addition, due to the influence of the excitation power, the Er3+ ions are in the unsaturated excitation state at this time. With the increase of the Er3+ ion doping concentration, the upconversion luminescence intensity of the sample was increased steadily. Afterward, as the doping concentration of Er3+ ions continues to be increased, the lattice sites of Y3+ ions around the uniformly distributed Er3+-1 ions in the matrix lattice are gradually replaced by Er3+-2 ions. The energy transfer distance between the Er3+-1–Er3+-2 ions is reduced,21 which results in the probability of cross-relaxation between neighboring ions being dramatically increased. The upconversion luminescence intensity of the sample is gradually reduced because the probability of non-radiative transitions in the sample is increased, and the probability of radiative transitions is decreased. Afterward, the Y3+ ion lattice sites around the Er3+-1 ions are gradually and uniformly occupied by more Er3+-2 ions as the Er3+ ion doping concentration continues to increase. At this point, the upconversion luminescence intensity of the samples begins to be gradually increased because the energy transfer effect between the Er3+-1–Er3+-2 ions begins to outweigh the cross-relaxation between Er3+-2–Er3+-1 ions. This will make the sample upconversion luminescence intensity begin to increase gradually, so the Er3+-1 ions are gradually saturating excited. When the doping concentration of Er3+ ions reaches 26 mol%, the energy transfer between Er3+-2 ions to Er3+-1 ions is greater than the cross-relaxation effect between the ions, reaching the maximum value, and the Er3+-1 ions in the energy receptor exhibit completely saturated excitation. Afterward, as the doping concentration of Er3+ ions continues to be increased, the cross-relaxation effect between neighboring ions begins to dominate so that the upconversion luminescence intensity of the sample is gradually reduced.22–24 This process is shown in Fig. 7.
As shown in Fig. 5, with the gradual increase in the doping concentration of Er3+ ions, the red light emission of the single-doped samples with Er3+ ions was substantially enhanced, and the green light emission was significantly reduced. Therefore, it is necessary to further analyze the upconversion of red emission and green emission of the samples from the perspective of energy level transition.
Fig. 8(a) shows the energy level transition of Er3+ ions under 1550 nm excitation, and Fig. 8(b) shows the IP curve under 1550 nm excitation, from which it can be seen that the energy level transition of Er3+ ions under 1550 nm excitation is a three-photon summation process. As for the self-absorption green light emission of Er3+ ions, the specific process is that the electrons of Er3+ ions in the ground state absorb three photons in succession to transit to the 2H11/2 energy level, after which some of the electrons in the 2H11/2 energy level directly relax to the ground state and produce green light emission at 525 nm. Secondly, some of the other electrons firstly relax to the 4S3/2 energy level and then return to the ground state by the radiative transition process, producing green light emission of 540 nm.
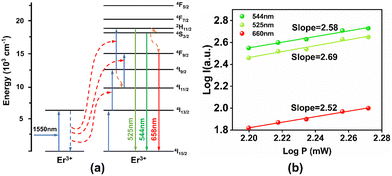 | ||
| Fig. 8 (a) Energy level transition of Er3+ ions under 1550 nm excitation; (b) IP curve of Er3+ ions under 1550 nm excitation. | ||
The reason for the self-absorbed red light emission for Er3+ ions is specified as follows. The electrons in the ground state of Er3+ ions absorb two photons consecutively to transit to the 4I9/2 energy level. Next, these electrons relax to the 4I11/2 energy level and then they absorb another photon and transit to the 4F9/2 energy level. Finally, these electrons relax to the ground state and produce red light emission at 660 nm. Meanwhile, the electrons of the Er3+ ions in the ground state absorb three photons directly to transit to the 2H11/2 energy level. Then they first relax to the 4S3/2 energy level and then relax to the 4F9/2 energy level. They eventually return to the ground state by the radiation transition process and produce the red light emission of the Er3+ ions.6,19,25,26
In summary, the Er3+ ions do not play a single role in the upconversion luminescence of the matrix lattice in the concentration-sequence doping experiments of the Er3+ ions. At low-concentration doping, the Er3+ ions mainly play the role of the energy acceptor of unsaturated excitation and the luminescence center of the matrix lattice, which leads to the first inflection point of concentration sequence doping. When the doping concentration of Er3+ ions is increased, some of the Er3+ ions gradually play the roles of energy carriers and energy transfer centers. However, the other part of the Er3+ ions play the role of an energy acceptor and saturation excitation center. This creates a second inflection point in the ion concentration doped series of samples. Therefore, we will use the saturation excitation of the Er3+ ion concentration as the basis for subsequent studies.
3.3 Modulating effect of the Er3+–Ho3+ ion dopant on the upconversion luminescence properties
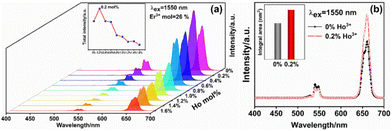
Currently, studies on the 1550 nm band are mainly focused on the Er3+ ion single-doped samples, and there are fewer studies on the response of other ions to the 1550 nm band.27–29 However, in recent years, it has been found that in the matrix lattice doped with Er3+ ions, the upconversion luminescence intensity of the samples can be greatly enhanced by doping with an appropriate amount of other ions.29,30 In this section, based on the above Er3+ ion doping of 26 mol%, the effect of this ion doping on the upconversion luminescence performance of the samples was investigated. As shown in Fig. 9(a), the upconversion luminescence intensity of the samples increased and then decreased when Ho3+ ions were doped into the matrix lattice of single-doped Er3+ ions. When the doping concentration of Ho3+ ions is 0.2 mol%, the upconversion luminescence intensity of the Er3+–Ho3+ ion co-doped system reaches the optimum, which is enhanced 1.23 times compared with the undoped Ho3+ ion sample, as shown in Fig. 9(b). When the Ho3+ ion doping concentration is further increased, the red light emission of the samples gradually dominated, and the green light emission of the samples was significantly quenched. This phenomenon is mainly caused by the different roles played by Ho3+ ions in the Er3+–Ho3+ co-doped system.The intrinsic mechanism of upconversion luminescence enhancement and energy transfer is analyzed in more detail. It is first necessary to determine whether the Ho3+ ions can act as an independent 1550 nm response upconversion absorption and emission center in the Er3+–Ho3+ ion co-doped system. Fig. 10(a) is the upconversion emission spectrum of the Ho3+ ion single-doped and Ho3+–Yb3+ ion co-doped samples under 1550 nm excitation. Fig. 10(b) shows the absorption spectra of mono-doped with Ho3+ ions and mono-doped samples with different concentrations of Er3+ ions. As can be seen from the figure, 1550 nm photons are significantly absorbed by the Ho3+ ion single-doped samples. The upconversion luminescence of the samples is composed of red and green emission, and the total luminescence intensity is dominated by red emission. For the Ho3+–Yb3+ ion co-doped samples, the luminescence of the samples still consists of red and green emission, whereas the total luminescence intensity is still dominated by red emission. Both the red and green light emission of the sample is reduced by doping with Yb3+ ions compared to the single-doped samples with Ho3+ ions. However, since there are almost no studies on the 1550 nm band that can be well responded to by other lanthanide activator ions, the current mechanism of upconversion energy level transition of Ho3+ ions under 1550 nm excitation is not well developed. In this section, the energy of each spectral term of Ho3+ ions was determined by reviewing the relevant literature, as shown in Table 2. After that, the energy difference between each spectral term was calculated: ΔE1 = 5049 cm−1 (5I8 → 5I7), ΔE2 = 6061 cm−1 (5I7 → 5I5), ΔE3 = 4264 cm−1 (5F5 → 5I5), ΔE4 = 5289 cm−1 (5F4 → 5I4).31 By comparing with the energy carried by 1550 nm photons (ΔE1550 nm = 6452 cm−1) and combining with the IP curves of 1550 nm excitation of single-doped Ho3+ ions, as shown in Fig. 11(a), the upconversion energy level transition diagrams of the Ho3+ ions excited at 1550 nm were determined, as shown in Fig. 11(b). The absorption of 1550 nm photons by Ho3+ ions makes Ho3+ ions play more roles in the Er3+–Ho3+ ion co-doping system. Firstly Ho3+ ions can be self-absorbing emission centers in the matrix lattice. Since the characteristic emissions of Er3+ ions and Ho3+ ions are highly overlapped in the visible region,29 the upconversion emission spectra of Er3+–Ho3+ ion co-doped samples are generated by the superposition of the characteristic emissions of both, which enhances the upconversion luminescence of the samples.29 Secondly, Ho3+ ions can also act as a reverse energy transfer center in the matrix lattice. The photon energy absorbed by the Er3+ ions is first temporarily stored by the Ho3+ ions. Then, the energy is transferred back to the Er3+ ions again by the reverse energy transfer process. Through this process, some of the Er3+ ions are excited twice, and the upconversion luminescence of the sample is enhanced.28,32 Finally, the Ho3+ ions can also act as an energy receptor for the Er3+ ions. The energy transferred from the Er3+ ions is absorbed by these Ho3+ ions and produces their characteristic emission, causing the upconversion luminescence of the Er3+–Ho3+ ion co-doped sample to be to enhanced.
| Energy level | 5I7 | 5I6 | 5I5 | 5I4 | 5F5 | 5S2 | 5F4 |
|---|---|---|---|---|---|---|---|
| ΔE (cm−1) | 5049 | 8550 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 110 110 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 155 155 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 374 374 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 325 325 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 444 444 |
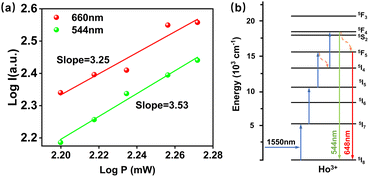 | ||
| Fig. 11 (a) IP curves of singly doped samples with Ho3+ ions under 1550 nm excitation. (b) Upconversion energy level transition diagram of Ho3+ ions under 1550 nm excitation. | ||
In summary, combining the upconversion emission spectra of the samples of the Er3+–Ho3+ ion co-doped system, it can be seen that the addition of the green emission of Er3+ ions and Ho3+ ions in the visible region produces the upconversion green emission of the samples. Fig. 12(a) shows the energy level transition of the Er3+–Ho3+ ion co-doped sample under 1550 nm excitation, and Fig. 12(b) shows the IP curve of the Er3+–Ho3+ ion co-doped sample under 1550 nm excitation. It can be seen that the energy level transition of the Er3+–Ho3+ ion co-doping system under 1550 nm excitation is a three-photon summation process. For the green emission of Er3+ ions of the samples, due to the doping of Ho3+ ions, the green emission pathway of Er3+ ions is increased by the reverse energy transfer process between Er3+–Ho3+ ions. The process is that the electrons of the Er3+ ions in the 4S3/2 energy level transfer to the neighboring Ho3+ ions by the energy transfer process. Then, these electrons are stored on the 5F4 energy level of the Ho3+ ions, after which they return to the 4S3/2 energy level of the Er3+ ions by the reverse energy transfer process. Finally, these electrons relax to the ground state and produce green light at 544 nm.28,29,33
 | ||
| Fig. 12 (a) Energy level transition of Er3+–Ho3+ ions co-doped under 1550 nm excitation. (b) IP curve of Er3+–Ho3+ ions co-doped under 1550 nm excitation. | ||
As for the upconversion green light emission of Ho3+ ions, it mainly consists of three parts, the first part is the self-absorption green light emission of Ho3+ ions, and the second part is the green light emission generated by the reverse energy transfer process between Ho3+–Er3+ ions. The third is the green light emission generated by the energy transfer process between Er3+–Ho3+.
For the self-absorbed green light emission of Ho3+ ions, the main process is that the electrons of the Ho3+ ions in the ground state absorb three photons consecutively to transit to the 5F5 energy level, after which they relax to the 5I4 energy level. Then these electrons absorb another photon to transit to the 5F4 energy level. Finally, they return to the ground state by a radiative transition process and produce the green light emission of the Ho3+ ions at 544 nm, and this process is shown in Fig. 13(a).
 | ||
| Fig. 13 (a) Schematic diagram of the reverse energy transfer of Er3+–Ho3+ ions. (b) Schematic diagram of the energy transfer of Er3+–Ho3+ ions. | ||
For the green light emission of the Ho3+ ions generated by the reverse energy transfer process between Ho3+–Er3+, the specific process is that the electrons in the 5F5 energy level of the Ho3+ ions transfer to the 4F9/2 energy level of the neighboring Er3+ ions by an energy transfer process. These electrons return to the 5F5 energy level of the Ho3+ ions by the reverse energy transfer process and further relax to the 5I4 energy level. Next these electrons absorb a photon to transit to the 5F4 energy level. Finally, they return to the ground state by the radiative transition process and produce green light emission of the Ho3+ ions. On the other hand, the electrons in the 5F4 energy level of the Ho3+ ions can also be transferred to the 4S3/2 energy level of the neighboring Er3+ ions through an energy transfer process and return to the 5F4 energy level of the Ho3+ ions by the reverse energy transfer process. They eventually relax to the ground state and produce green light emission of the Ho3+ ions, and this process is shown in Fig. 13(a).28,29
The reason for the green light emission generated by the energy transfer process between Er3+–Ho3+ ions is specified as follows, the electrons in the 4I13/2 energy level of Er3+ ions transfer to the neighboring Ho3+ ions by the energy transfer process, keeping these electrons in the 5I7 energy level of Ho3+ ions. Meanwhile, the electrons at the 5I7 energy level absorb two photons consecutively to transit to the 5F5 energy level, and they relax to the 5I4 energy level. And then, these electrons absorb a photon to transit to the 5F4 energy level. Finally, they return to the ground state by the radiative transition process and produce green light emission of Ho3+ ions, and this process is shown in Fig. 13(b).28,29,34
For the Er3+–Ho3+ ion co-doped system under 1550 nm excitation, the red light emission is also generated by summating the red light emission of Er3+ ions and the Ho3+ ions in the visible region. For the red emission of Er3+ ions of the samples, due to the doping of Ho3+ ions, the red emission pathway of Er3+ ions is increased by the reverse energy transfer process between Er3+–Ho3+ ions. The specific process is that the electrons of the Er3+ ions in the 4S3/2 energy level transfer to the neighboring Ho3+ ions by the energy transfer process, and these electrons return to the 4S3/2 energy level of the Er3+ ions by the reverse energy transfer process. Then these electrons relax to the 4F9/2 energy level. Finally, they return to the ground state by the radiative transition process and produce red light emission of the Er3+ ions. On the other hand, the electrons in the 4F9/2 energy level of the Er3+ ions can also transfer to the 5F5 energy level of the neighboring Ho3+ ions by the energy transfer process, and these electrons return to the 4F9/2 energy level by the reverse energy transfer process. Finally, they relax to the ground state and produce the red light emission of the Er3+ ions, and this process is shown in Fig. 13(a).28,29,35
As for the upconversion red light emission of Ho3+ ions, it mainly consists of three parts, the first is the self-absorption red light emission of Ho3+ ions, and the second is the red light emission generated by the reverse energy transfer process between Ho3+–Er3+ ions. The third is the red light emission generated by the energy transfer process between Er3+–Ho3+ ions.
For the self-absorbed red light emission of Ho3+ ions, the main process is that the electrons of the Ho3+ ions in the ground state absorb three photons consecutively to transit to the 5F5 energy level. Then they immediately relax to the ground state and produce the red light emission of the Ho3+ ions. On the other hand, the electrons of the Ho3+ ions in the 5F5 energy level relax to the 5I4 energy level, and absorb another photon to transit to the 5F4 energy level. Finally, they return to the ground state by a radiative transition process and produce the red light emission of the Ho3+ ions.
For the red light emission of the Ho3+ ions generated by the reverse energy transfer process between Ho3+–Er3+, the specific process is that the electrons in the 5F5 energy level of the Ho3+ ions can transfer to the 4F9/2 energy level of the neighboring Er3+ ions by an energy transfer process. After that, these electrons return to the 5F5 energy level of the Ho3+ ions by the reverse energy transfer process. Then, they return to the ground state by the radiative transition process and produce red light emission of the Ho3+ ions, and this process is shown in Fig. 13(a).28,29,35
The reason for the red light emission generated by the energy transfer process between Er3+–Ho3+ ions is specified as follows. The electrons in the 4I13/2 energy level of Er3+ ions transfer to the neighboring Ho3+ ions by the energy transfer process, keeping these electrons in the 5I7 energy level of Ho3+ ions. Then, these electrons at the 5I7 energy level absorb two photons consecutively to transit to the 5F5 energy level. They relax to the ground state and produce red light emission of Ho3+ ions. On the other hand, the electrons of the Er3+ ions in the 4F9/2 energy level transfer to the 5F5 energy level of the neighboring Ho3+ ions by an energy transfer process, and then they return to the ground state by a radiative transition process and produce the red light emission of the Ho3+ ions; this process is shown Fig. 13(b).28,29,35
Fig. 14 shows the fluorescence lifetime decay curves of the Er3+–Ho3+ ion co-doped samples under 980 nm excitation. The graph shows that the co-doped Er3+–Ho3+ will lead to a certain degree of enhancing the fluorescence lifetime of 540 nm. This is mainly due to the doping of Ho3+ ions, which makes the electrons on the 2H11/2 and 4S3/2 energy levels of the Er3+ ions sustain a longer period. This decreases the chance of electrons at this energy level returning to the ground state. Therefore, the fluorescence weakening of 540 nm will appear in the upconversion emission spectrum. On the contrary, doping the Er3+ ions with the appropriate amount of Ho3+ ions will result in a substantial enhancement of the red emission of the samples. And the fluorescence lifetime of 660 nm is reduced after being doped with Ho3+ ions. This is mainly due to many electrons preferentially returning to the ground state and producing the red light emission of 660 nm. Therefore, all these factors increase the red lifetime of Er3+–Ho3+ ion co-doped samples. The reduction of 660 nm lifetime also corroborates the significant enhancement of the red emission presented by the emission spectrum.36
The fluorescence decay curves are experimental evidence of energy transfer between rare earths. In order to further clarify the energy transfer mechanism of Ho3+ ion doping, we calculated the energy transfer efficiency of Er3+ ions co-doped with Er–Ho samples as shown in eqn (3):
 | (3) |
Since in the Er3+–Ho3+ ion co-doped system, the doping amount of Ho3+ ions is extremely small compared to the optimal doping concentration of Er3+ ions, the contribution of the characteristic emission of Ho3+ ions to the upconversion luminescence of the sample is extremely weak. Therefore, the upconversion luminescence of the sample is still dominated by the luminescence of Er3+ ions. As compared to the increase in the green emission pathway of the Er3+–Ho3+ ion co-doped sample, the red light emission pathway is significantly more than the green light emission. Due to the combined effect of cross-relaxation and energy transfer between the ions, the chance of the electron population to the green light energy level is greatly reduced. So the green light emission of the Er3+–Ho3+ co-doped samples is significantly quenched, while the red light emission is substantially enhanced.
3.4 Modulating the effect of Er3+–Ho3+–Yb3+ ion dopants on the upconversion luminescence properties
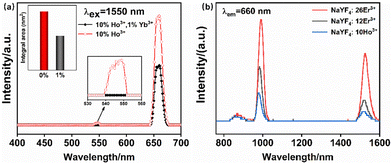
In the above study, the role played by Ho3+ ions in the Er3+–Ho3+ co-doping system and the enhancement of upconversion luminescence was investigated. Afterward, it was found that the up-conversion luminescence of the samples doped with Er3+ ions was significantly enhanced after doping with Yb3+ ions. Combined with the above Ho3+–Yb3+ ion co-doping experiment, the doping of Yb3+ ions can make the Ho3+ ions continue to produce the characteristic red and green light emission, but the upconversion luminescence of Ho3+ ions is reduced under the excitation at 1550 nm. This may be due to the large energy difference between the 2F5/2 energy level of the Yb3+ ion and the 5I6 energy level of the Ho3+ ions.37 The reverse energy transfer process between the Ho3+–Yb3+ ion was difficult to occur. Therefore, the doping of Yb3+ ions depletes the energy absorbed by Ho3+ ions, the upconversion luminescence intensity of the Ho3+–Yb3+ co-doped system was reduced. Consequently, in this section, the appropriate amount of Yb3+ ions based on the optimal doping concentration of Er3+–Ho3+ ions mentioned above was doped, and the role of Yb3+ ions in the multi-ion co-doped system and the effect on the upconversion luminescence performance of the samples was investigated.Fig. 15 shows the upconversion emission spectra of the Er3+–Ho3+–Yb3+ ion triple-doped samples under 1550 nm excitation. As can be seen from the figure, as the doping concentration of Yb3+ ions was increased, the upconversion luminescence intensity of the samples increases firstly and then decreases. When the Yb3+ ion doping concentration is 1 mol%, the upconversion luminescence intensity of the sample reaches the best value. The red and green emission intensities of the samples are enhanced to a certain extent after the doping of Yb3+ ions in the matrix lattice. Since the photons of 1550 nm are not absorbed by Yb3+ ions, the photons in the Er3+–Ho3+–Yb3+ ion doped samples are mainly accomplished by Er3+ ions, and a small portion of the photons are absorbed by Ho3+ ions.29,30 By reviewing the relevant literature and combining the experimental phenomena, it can be seen that Yb3+ ions for the Er3+–Ho3+–Yb3+ triple-doped system of upconversion luminescence mainly plays the role of a reverse energy transfer center of Er3+–Yb3+ ions. This process means that the utilization rate of the Er3+ ions for the photons is increased so that the intensity of the upconversion luminescence is significantly increased. The energy transfer between the Ho3+–Yb3+ ions leads to rapid energy depletion, so the upconversion luminescence intensity of the Ho3+–Yb3+ co-doped samples is significantly quenched.
Fig. 16(a) shows the energy level transition of the Er3+–Ho3+–Yb3+ion triple-doped sample under 1550 nm excitation, and Fig. 16(b) shows the IP curve of the Er3+–Ho3+–Yb3+ion triple-doped sample under 1550 nm excitation, from which it can be seen that the energy level transition of the Er3+–Ho3+–Yb3+ion triple-doped system under 1550 nm excitation is a three-photon summation process. For the Er3+–Ho3+–Yb3+ triple-doped system under 1550 nm excitation, the increased green emission pathway due to the doping of Yb3+ ions mainly consists of the green emission of Er3+ ions. The specific process is that the electrons of the Er3+ ions in the ground state absorb two photons in succession to transit to the 4I9/2 energy level, after which they relax to the 4I11/2 energy level. Then, these electrons transfer to the neighboring Yb3+ ions by the energy transfer process and keep the electrons in the 2F5/2 energy level of the Yb3+ ions. And then, these electrons return to the neighboring Er3+ ions by a reverse energy transfer process, making the electrons in the 4I11/2 energy level directly transit to the 4F7/2 energy level, after which these electrons relax to the 2H11/2 energy level and the 4S3/2 energy level. Finally, they return to the ground state by the radiative transfer process and produce green light emission of the Er3+ ions.38–40
 | ||
| Fig. 16 (a) Energy level transition of Er3+–Ho3+–Yb3+ion triple-doping under 1550 nm excitation. (b) IP curve of Er3+–Ho3+–Yb3+ion triple-doping under 1550 nm excitation. | ||
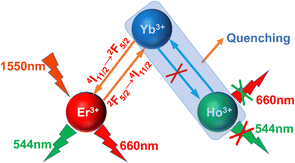
For 1550 nm excitation in the Er3+–Ho3+–Yb3+ triple-doped system, the red light emission pathway is increased due to the doping of Yb3+ ions. This mainly originates from the reverse energy transfer process of Er3+–Yb3+ ions. The specific process is that the electrons of the Er3+ ions in the ground state continuously absorb two photons to transit to the 4I9/2 energy level, and then they relax to the 4I11/2 energy level. Then these electrons are transferred to the 2F5/2 energy level of the neighboring Yb3+ ions by the energy transfer process, and afterward they return to the 4I11/2 energy level of the Er3+ ions by the reverse energy transfer process. These electrons absorb a photon to transit to the 4F9/2 energy level, and they eventually return to the ground state by the radiation transition process and produce red light emission of the Er3+ ions. Finally, the electrons at the 2H11/2 energy level with the electrons on the 4I11/2 energy level of the Er3+ ions can undergo a cross-relaxation process, making the electrons at the 4I11/2 energy level transit to the 4F9/2 energy level, after which these electrons return to the ground state by the radiation transition process and produce the red light emission of the Er3+ ions.36,41–43Fig. 17 shows the schematic diagram of the reverse energy transfer between Yb3+ ions and Er3+ ions and Ho3+ ions. Through the above experimental results, we only observed the reverse energy transfer process between Er3+–Yb3+ ions; the reverse energy transfer phenomenon between Ho3+–Yb3+ ions did not occur. Therefore, for the Er3+–Yb3+ ion pair, the up-conversion luminescence intensity of the sample is significantly enhanced because the process can significantly increase the utilization rate of Er3+ ions for 1550 nm photons.44–53 Since we do not observe the reverse energy transfer between Ho3+–Yb3+ ion pairs, the up-conversion luminescence intensity of the samples was significantly quenched due to the cross-relaxation between the Ho3+–Yb3+ ions. The schematic diagram of the reverse energy transfer between the ions in Fig. 17 can well summarize the process of the reverse energy transfer between the Er3+–Ho3+–Yb3+ ions.
In summary, for the upconversion luminescence of the Er3+–Ho3+–Yb3+ multi-ion co-sensitized samples, the upconversion red and green emissions of the samples are generated by the summation of the characteristic emissions of the Er3+ ions and Ho3+ ions in the visible region. Since the doping concentration of Er3+ ions is much larger than that of Ho3+ ions, the contribution to the upconversion luminescence intensity of the triple-doped Er3+–Ho3+–Yb3+ system is still dominated by Er3+ ions. The Ho3+ ions play the energy transfer capture center role in the upconversion luminescence of the samples. Meanwhile, they may also be part of the self-absorption activation center and the energy receptor luminescence center of the Er3+ ions. The doping of Yb3+ ions mainly plays the role of the reverse energy transfer center between Er3+–Yb3+ ion pairs. Although the doping of Yb3+ ions in the Ho3+–Yb3+ co-doped samples significantly reduces the up-conversion luminescence intensity of the samples, the doping concentration of Ho3+ ions in the Er3+–Ho3+–Yb3+ triple-doped system is very small. Therefore, Yb3+ ions have little effect on the characteristic emission of Ho3+ ions in the triple-doped samples. Meanwhile, Ho3+ ions and Yb3+ ions can act as energy transient capture centers and reverse energy transfer centers in the matrix lattice structure, respectively. And the energy that should be dissipated in the energy transfer and electron transition process will be stored temporarily, which increases the ions’ utilization rate for photons. Absorption of 1550 nm photons by Ho3+ ions results in spectral addition. In summary, these factors can enhance the upconversion emission spectral intensity of the samples.
4. Conclusions
Er3+–Ho3+–Yb3+ triple-doped fluoride up-conversion phosphors were successfully prepared by the low-temperature combustion synthesis method. It is found that in the series of doping concentrations of Er3+ ions under 1550 nm excitation, when the doping concentration of Er3+ ions is 12 mol%, due to the limitation of excitation power, Er3+-1 ions mainly play the role of unsaturated excitation centers in the matrix lattice. When the doping concentration of Er3+ ions continues to increase until it reaches 26 mol%, Er3+-2 ions will be uniformly distributed around the Er3+-1 ions, playing the role of a sensitizer in the matrix lattice, and saturation excitation of the Er3+-1 ions will significantly enhance the upconversion of the luminescence intensity. The upconversion luminescence intensity of the Er3+–Ho3+ co-doped samples is enhanced when a small amount of Ho3+ ions are doped into the matrix lattice. The upconversion luminescence intensity of the samples reaches the optimal value when the doping concentration of Ho3+ ions is 0.2 mol%, and the doping of Ho3+ ions substantially enhances the red emission of the samples and bursts the green light emission. At this time, Ho3+ ions can firstly play the roles of transient energy capture centers and reverse energy transfer centers in the matrix lattice, and secondly also act as the self-absorption luminescence center in the matrix lattice. The upconversion luminescence intensity of the Er3+–Ho3+–Yb3+ triple-doped system reaches the optimal value when 1 mol% of Yb3+ ions is doped in the Er3+–Ho3+ co-doped sample. At this time, Yb3+ ions mainly play the role of the reverse energy transfer center between Er3+–Yb3+ ion pairs in the matrix lattice. This study has significant applications in enhancing the upconversion luminescence of the samples and displaying high color purity in the 1550 nm band.Author contributions
Bohan Lei: conceptualization, data curation, formal analysis, investigation, writing original draft. Liping Lu: supervision, writing – review & editing. Haiying Sun: formal analysis, investigation.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the Application Innovation Program of the Equipment pre-research (627011103) and the Jilin Province Education Department Project (grant no. JJKH20200760KJ).Notes and references
- X. Zhu, Q. Su and W. Feng, et al., Anti-Stokes shift luminescent materials for bio-applications, Chem. Soc. Rev., 2017, 46(4), 1025–1039 RSC.
- N. Li, N. Eedugurala and D. S. Leem, et al., Organic upconversion imager with dual electronic and optical readouts for shortwave infrared light detection, Adv. Funct. Mater., 2021, 31(16), 2100565 CrossRef CAS.
- L. Gao, X. Shan and X. Xu, et al., Correction: Video-rate upconversion display from optimized lanthanide ion doped upconversion nanoparticles, Nanoscale, 2020, 12(36), 18987 RSC.
- F. Jia, G. Li and B. Yang, et al., Investigation of rare earth upconversion fluorescent nanoparticles in biomedical field, Nanotechnol. Rev., 2019, 8(1), 1–17 CAS.
- W. Wang, J. Tian and J. Dong, et al., Growth, spectroscopic properties and up-conversion of Yb, Pr co-doped CaF2 crystals, J. Lumin., 2021, 233, 117931 CrossRef CAS.
- H. N. Luitel, S. Mizuno and Y. Takeda, CaTiO3:Er3+, Ni2+ broadband-sensitive upconverter: an effective way to harvest unused NIR solar irradiation for crystalline silicon solar cells, Phys. Status Solidi A, 2017, 214(8), 1600899 CrossRef.
- Z. Li, H. Yuan and W. Yuan, et al., Upconversion nanoprobes for biodetections, Coord. Chem. Rev., 2018, 354, 155–168 CrossRef CAS.
- X. Yin, W. Hong and X. Mingming, et al., Upconversion luminescence of Y2Ti2O7:Er3+ under 1550 and 980 nm excitation, J. Rare Earths, 2017, 35(3), 230–234 CrossRef CAS.
- N. Y. Mostafa, A. Badawi and S. I. Ahmed, Influence of Cu and Ag doping on structure and optical properties of In2O3 thin film prepared by spray pyrolysis, Results Phys., 2018, 10, 126–131 CrossRef.
- H. Bian, Y. Liu and D. Yan, et al., Light-induced electrons suppressed by Eu3+ ions doped in Ca11.94−xSrxAl14O33 caged phosphors for LED and FEDs, J. Am. Ceram. Soc., 2017, 100(8), 3467–3477 CrossRef CAS.
- C. G. Pope, X-ray diffraction and the Bragg equation, J. Chem. Educ., 1997, 74(1), 129 CrossRef CAS.
- Y. Liu, Y. Liu and M. G. B. Drew, Comparison of calculations for interplanar distances in a crystal lattice, Crystallogr. Rev., 2017, 23(4), 252–301 CrossRef.
- K. N. Nicholson and S. A. Wood, Aqueous Geochemistry of Rare Earth Elements and Yttrium. XII: Potentiometric Stability Constant Determination of Bis -Tris Complexes with La, Nd, Eu, Gd, Yb, Dy, Er, Lu, and Y, J. Solution Chem., 2002, 31(9), 703–717 CrossRef CAS.
- S.-O. Yoon, C.-B. Hong and S. Kim, Microwave dielectric properties of Na+ and M3+-doped Ba(Mg0.5W0.5)O3 ceramics, J. Electroceram., 2018, 41, 16–22 CrossRef CAS.
- A. Ghafoor, M. A. Khan and M. U. Islam, et al., Structural and electromagnetic studies of Ni0.7Zn0.3Ho2xFe2−2xO4 ferrites, Ceram. Int., 2016, 42(12), 14252–14256 CrossRef CAS.
- W. S. Silveira and A. J. S. Silva, Nascimento P a M D, et al. Improving the luminescence properties of YAG:Ce3+ phosphors by co-doping Sr2+ ions, Optik, 2021, 231, 166363 CrossRef CAS.
- Y. Chen, F. Peng and Q. Zhang, et al., Growth, structure and spectroscopic properties of 1 at% Er3+:GdTaO4 laser crystal, J. Lumin., 2017, 192, 555–561 CrossRef CAS.
- M. Li, S. Sun and L. Zhang, et al., Growth and spectral properties of a promising laser crystal Yb3+/Er3+:Ca9La(VO4)7, J. Cryst. Growth, 2016, 451, 52–56 CrossRef CAS.
- C. Xu, Q. Yang and G. Ren, et al., Pure red upconversion emission from Yb3Al5O12 phase doped with high Er3+ concentration, J. Alloys Compd., 2010, 503(1), 82–85 CrossRef CAS.
- H. Krzyżanowska, Y. Fu and K. S. Ni, et al., Efficient Energy Transfer between Si Nanostructures and Er Located at a Controlled Distance, ACS Photonics, 2016, 3(4), 564–570 CrossRef.
- N. V. Kononets, V. V. Seminko and P. O. Maksimchuk, et al., Processes of energy migration in mixed europium–lanthanum magnesium borate nanocrystals, Spectrosc. Lett., 2017, 50(7), 399–403 CrossRef CAS.
- H. Hanchang, Z. Yanyi and L. Mingchen, et al., The effect of Er3+ concentration on the kinetics of multiband upconversion in NaYF4:Yb/Er microcrystals, Front. Chem., 2023, 11, 1097250 CrossRef PubMed.
- L. Yan, B. Zhou and N. Song, et al., Self-sensitization induced upconversion of Er3+ in core–shell nanoparticles, Nanoscale, 2018, 10(37), 17949–17957 RSC.
- C. Lee, et al., Origin of strong red emission in Er3+-based upconversion materials: role of intermediate states and cross relaxation, Phys. Chem. Chem. Phys., 2019, 21(43), 24026–24033 RSC.
- A. R. Hong, J.-H. Kyhm and G. Kang, et al., Orthogonal R/G/B Upconversion Luminescence-based Full-Color Tunable Upconversion Nanophosphors for Transparent Displays, Nano Lett., 2021, 21(11), 4838–4844 CrossRef CAS PubMed.
- G. A. Kumar, M. Pokhrel and D. K. Sardar, Intense visible and near infrared upconversion in M2O2S:Er (M = Y, Gd, La) phosphor under 1550 nm excitation, Mater. Lett., 2012, 68, 395–398 CrossRef CAS.
- W. Wang, Z. Feng and B. Li, et al., Er3+ self-sensitized nanoprobes with enhanced 1525 nm downshifting emission for NIR-IIb in vivo bio-imaging, J. Mater. Chem. B, 2021, 9(12), 2899–2908 RSC.
- Y. Bowen, G. Linna and L. Tiesheng, Nearly pure red up-conversion emission of Ba4Bi3F17:Ln3+ with 1550 nm wavelength excitation by controlling the doping ions, Opt. Mater., 2022, 125, 112076 CrossRef.
- X. Cheng, H. Ge and Y. Wei, et al., Design for Brighter Photon Upconversion Emissions via Energy Level Overlap of Lanthanide Ions, ACS Nano, 2018, 12(11), 10992–10999 CrossRef CAS PubMed.
- X. Cheng, Y. Pan and Z. Yuan, et al., Er3+ Sensitized Photon Upconversion Nanocrystals, Adv. Funct. Mater., 2018, 28(22), 1800208 CrossRef.
- A. Y. Freidzon, I. A. Kurbatov and V. I. Vovna, Ab initio calculation of energy levels of trivalent lanthanide ions, Phys. Chem. Chem. Phys., 2018, 20(21), 14564–14577 RSC.
- Y. Wei, C. Su and H. Zhang, et al., Color-tunable up-conversion emission from Yb3+/Er3+/Tm3+/Ho3+ codoped KY(MoO4)2 microcrystals based on energy transfer, Ceram. Int., 2016, 42(4), 4642–4647 CrossRef CAS.
- X. Zhou, G. Ju and T. Dai, et al., Investigation of new color-tunable up-conversion phosphors and their long-persistent luminescence properties for potential biomedical applications, Appl. Phys. A: Mater. Sci. Process., 2019, 125, 1–8 CrossRef CAS.
- K. Lemański, R. Pązik and P. J. Dereń, Efficient up-conversion emission and energy transfer in LaAlO3 doped with Er3+, Ho3+, and Yb3+ ions, Opt. Mater., 2012, 34(12), 1990–1993 CrossRef.
- S. Lu, S. Yao and Q. Chen, et al., Intense red upconversion emission and energy transfer in Yb3+/Ho3+/Er3+:CaYAlO4, J. Lumin., 2018, 196, 36–39 CrossRef CAS.
- S. Balaji, D. Ghosh and K. Biswas, et al., Insights into Er3+ ↔ Yb3+ energy transfer dynamics upon infrared ∼1550 nm excitation in a low phonon fluoro-tellurite glass system, J. Lumin., 2017, 187, 441–448 CrossRef CAS.
- S. H. Jeong, Y. K. Kshetri and S. H. Kim, et al., Microstructure investigation and multicolor upconversion in Yb3+/Ln3+(Ln = Er/Tm/Ho) ions doped α-Sialon, Prog. Nat. Sci.: Mater. Int., 2019, 29(5), 549–555 CrossRef CAS.
- A. A. Lyapin, S. V. Gushchin and S. V. Kuznetsov, et al., Infrared-to-visible upconversion luminescence in SrF2:Er powders upon excitation of the 4I13/2 level, Opt. Mater. Express, 2018, 8(7), 1863–1869 CrossRef CAS.
- X. Yin, H. Wang and T. Jiang, et al., Up-conversion luminescence properties and thermal effects of LaVO4:Er3+ under 1550 nm excitation, Mater. Res. Bull., 2017, 86, 228–233 CrossRef CAS.
- R. Lei, H. Wang and S. Xu, et al., Combustion synthesis and enhanced 1.5 μm emission in Y2O3:Er3+ powders codoped with La3+ ions, J. Rare Earths, 2016, 34(2), 125–129 CrossRef CAS.
- W. Yu, Y. Tian and M. Xing, et al., Up-conversion luminescence of NaY(WO4)2:Yb, Er under 1550 and 980 nm excitation, Mater. Res. Bull., 2016, 80, 223–229 CrossRef CAS.
- A. A. Lyapin, P. A. Ryabochkina and S. V. Gushchin, et al., Upconversion Luminescence of Fluoride Phosphors SrF2:Er, Yb under Laser Excitation at 1.5 μm, Opt. Spectrosc., 2018, 125, 537–542 CrossRef CAS.
- H. Wang, M. Xing and X. Luo, et al., Upconversion emission colour modulation of Y2O2S:Yb, Er under 1.55 μm and 980 nm excitation, J. Alloys Compd., 2014, 587, 344–348 CrossRef CAS.
- Chiho Lee, et al., Origin of strong red emission in Er3+-based upconversion materials: role of intermediate states and cross relaxation, Phys. Chem. Chem. Phys., 2019, 21(43), 24026–24033 RSC.
- Hong Wang, et al., Designing Er3+ Single-doped ternary sulfide for highly efficient upconversion luminescence under 1550 nm excitation, Chem. Eng. J., 2023, 468, 143558 CrossRef CAS.
- H. Wang, et al., Upconversion emission colour modulation of Y2O2S:Yb, Er under 1.55 μm and 980 nm excitation, J. Alloys Compd., 2014, 587, 344–348 CrossRef CAS.
- A. Tymiński, R. M. Inocencio and G. Tomasz, Upconversion in Detail: Multicolor Emission of Yb/Er/Tm-Doped Nanoparticles under 800, 975, 1208, and 1532 nm Excitation Wavelengths, Part. Part. Syst. Charact., 2020, 37(8), 2000068 CrossRef.
- X. Yin, et al., Towards highly efficient NIR II response up-conversion phosphor enabled by long lifetimes of Er3+, Nat. Commun., 2022, 13(1), 6549 CrossRef CAS PubMed.
- Wen Xu, et al., Atomic-scale imaging of ytterbium ions in lead halide perovskites, Sci. Adv., 2023, 9(35), eadi7931 CrossRef CAS PubMed.
- Z. Li, G. Zhu and S. Li, et al., High-Performance NIR Emission in Chromium-Doped Garnet Phosphors Enabled by Structure and Excitation Regulation, Laser Photonics Rev., 2024, 18(1), 2300732 CrossRef CAS.
- Z. Li, G. Zhu and S. Li, et al., Ultra-small Stokes shift induced thermal robust efficient blue-emitting alkaline phosphate phosphors for LWUV WLEDs, Ceram. Int., 2023, 49(13), 21510–21520 CrossRef CAS.
- L. Yan, M. Xing and Y. Ma, et al., Promising lanthanide-doped double molybdates KYb(MoO4)2 phosphors for highly efficient upconversion luminescence and temperature sensing, Spectrochim. Acta, Part A, 2024, 308, 123751 CrossRef CAS PubMed.
- Y. Xu, M. Zou and H. Wang, et al., Upconversion nanoparticles@ single-walled carbon nanotubes composites as efficient self-monitored photo-thermal agents, Spectrochim. Acta, Part A, 2023, 303, 123173 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |