Detection of SO2 using a chemically stable Ni(II)-MOF†
Valeria B.
López-Cervantes‡
a,
Dae Won
Kim‡
b,
Juan L.
Obeso‡
 ac,
Eva
Martínez-Ahumada
a,
Yoarhy A.
Amador-Sánchez
a,
Elí
Sánchez-González
ac,
Eva
Martínez-Ahumada
a,
Yoarhy A.
Amador-Sánchez
a,
Elí
Sánchez-González
 a,
Carolina
Leyva
a,
Carolina
Leyva
 c,
Chang Seop
Hong
c,
Chang Seop
Hong
 *b,
Ilich A.
Ibarra
*b,
Ilich A.
Ibarra
 *a and
Diego
Solis-Ibarra
*a and
Diego
Solis-Ibarra
 *a
*a
aLaboratorio de Fisicoquímica y Reactividad de Superficies (LaFReS), Instituto de Investigaciones en Materiales, Universidad Nacional Autónoma de México, Circuito Exterior s/n, CU, Coyoacán, 04510, Ciudad de México, Mexico. E-mail: diego.solis@unam.mx; argel@unam.mx; Fax: +52 55 5622 4595
bDepartment of Chemistry, Korea University, Seoul 02841, Republic of Korea. E-mail: cshong@korea.ac.kr
cInstituto Politécnico Nacional, CICATA U. Legaria, Laboratorio Nacional de Ciencia, Tecnología y Gestión Integrada del Agua (LNAgua), Legaria 694 Irrigación, Miguel Hidalgo, 11500, CDMX, Mexico
First published on 13th July 2023
Abstract
The MOF-type Ni2(dobpdc) shows a high chemical stability towards SO2, high capacity for SO2 capture at low pressure (4.3 mmol g−1 at 298 K and up to 0.05 bar), and exceptional cycling performance. Fluorescence experiments demonstrated the SO2 detection properties of Ni2(dobpdc) with a remarkable SO2 detection selectivity. Finally, time-resolved photoluminescence experiments provided a plausible mechanism of SO2 detection by this Ni(II)-based MOF material.
Introduction
Sulphur dioxide (SO2) is classified as one of the most toxic chemicals with a pungent odour. SO2 is an irritant, colourless, irritating, and non-flammable gas easily absorbed by the respiratory system or dermal contact.1 The presence of SO2 is accountable for a direct rise in respiratory complications (e.g., broncho-constriction in lung function) and even death (contact over 100 ppm of SO2 in only a few minutes).2 Although it is naturally formed by volcanic activity, the main source of SO2 is the combustion of fossil fuels containing sulphur and metal extraction from ores.3 Moreover, SO2 is a precursor of particulate matter (PM), another high threat to human health.4 In addition, SO2 is one of the principal constituents of acid rain, which negatively impacts aquatic environments and causes loss of minerals and nutrients from the soil, hampering the growth of forests and crop plants.5 Acid rain, in urban areas, drastically accelerates the corrosion of metallic structures and attacks the main constituents of buildings (e.g., limestone, marble, and mortar).6 Thus, it becomes imperative to improve air quality, especially in urban areas, by reducing the emissions of SO2 and identifying potentially polluted environments with SO2.Most of the technological efforts toward SO2 have been concentrated on capturing this toxic gas. For this, the most used technology for SO2 capture is based on scrubbers.7 However, this technology has demonstrated serious disadvantages such as low capture of SO2, vast quantities of wastewater, corrosion of pipelines, and considerable cost of use and recovery.8 In addition, other solid-state materials (e.g., zeolites9 and metal oxides10) have been investigated for the efficient capture of SO2 with considerable drawbacks, such as high re-activation temperatures (above 250 °C) and a significant loss in porosity.11 Unquestionably, the capture of SO2 has shown to be a challenging task that requires new technological approaches.
A relatively new class of highly crystalline and porous materials known as metal–organic frameworks (MOFs) has been recently explored.12 Selected examples have demonstrated remarkable SO2 capture results,13 even under humid conditions.14 However, one of the main criticisms of MOFs is the current high cost of production for the organic ligands, in combination with difficult scalability, which raises questions about the sustainable economics of capturing SO2 using MOFs at the industrial scale.
Interestingly, more research needs to be dedicated to detecting SO2 with MOFs.15 Conversely to SO2 capture with MOFs, where large amounts of these materials are required, for the detection of SO2, only small amounts of a particular MOF material are needed.16 Thus, detecting SO2 is an extremely desirable characteristic for a MOF material to identify potentially polluted surroundings with this toxic gas. For example, if a chemically stable MOF material exhibits luminescence properties, the fluorescent response to SO2 change would be the key to exploring such material as an effective SO2 detector.
Thus, with this specific target in mind, a robust and chemically stable Ni(II)-based MOF material entitled Ni2(dobpdc) was selected to address SO2 detection effectively. Ni2(dobpdc) MOF is constructed from the coordination of Ni(II) ions and 4,4′-dioxidobiphenyl-3,3′-dicarboxylate (dobpdc)4− ligand (Fig. S1†).17 Each Ni(II) centre is hexa-coordinated to six O-donor atoms: in the equatorial plane, Ni(II) is coordinated to two trans-disposed bridgings (μ2) aryloxide O atoms from two different dobpdc ligands to one bridging (μ2) carboxylate O atom and one nonbridging carboxylate O atom. Also, from the ligand, whereas, in the axial plane, the Ni(II) centre is coordinated to one bridging (μ2) carboxylate O atom from the ligand and a methanol (MeOH) molecule, which is used as a solvent in the solvothermal synthesis.18 Such coordination array forms hexagonal helical chains across the c-axis of the crystal with a pore size of approximately 17 Å (Fig. S1†). After an activation process at 523 K for 12 h under vacuum (1.7 × 10−3 Torr), coordinated MeOH molecules to Ni(II) metal centres are fully removed, providing uncoordinated Ni2+ sites.
Herein, Ni2(dobpdc) exhibited to be chemically stable to SO2, with a total SO2 uptake of 12.5 mmol g−1, at 298 K and 1 bar. Remarkably, solid-state fluorescence experiments demonstrated that Ni2(dobpdc) has proven to be an effective selective detector of this toxic pollutant.
Powder X-ray diffraction (PXRD) and thermogravimetric analysis (TGA) confirmed the phase purity of Ni2(dobpdc) (Fig. S2 and S3†). A sample of Ni2(dobpdc) was activated at 523 K for 12 h under vacuum (1.4 × 10−3 torr). Then, an N2 isotherm at 77 K (Fig. S4†) demonstrated a BET surface area of 3005 m2 g−1 with a pore volume of 1.11 cm3 g−1.
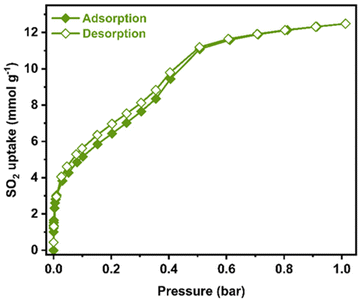
SO2 adsorption–desorption isotherm using a Dynamic Gravimetric Gas/Vapour Sorption Analyser, DVS vacuum (Surface Measurement Systems Ltd), was carried out from 0 to 1 bar at 298 K on an activated sample of Ni2(dobpdc). Fig. 1 shows the resulting isotherm obtained. A steep with fast SO2 uptake from 0.0 to 0.05 bar is noted, accounting for a total uptake of approximately 4.3 mmol g−1. From 0.05 to 0.4 bar, the SO2 adsorption isotherm showed an approximate linear uptake with a total amount of ≈ 9.4 mmol g−1. Finally, from 0.4 to 1.0 bar (end of the SO2 adsorption experiment), a slow SO2 uptake is observed. A total SO2 uptake of 12.5 mmol g−1 was achieved. This fully SO2 uptake is comparable to representative chemically stable MOFs.19 Furthermore, the desorption isotherm shows a slight hysteresis, suggesting a relatively high SO2/MOF interaction energy.
Possibly, the most significant property of a chemically stable MOF material toward SO2 detection is high SO2 adsorption at low pressure (P < 0.1 bar). Considering that concentration ranges for SO2 detection are at the ppm level, these can be naturally correlated to the SO2 low-pressure range. Thus, the total SO2 uptake (at ambient pressure) becomes irrelevant. For example, flue gas displays SO2 concentrations up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm, which is directly related to a pressure below 0.05 bar.20
000 ppm, which is directly related to a pressure below 0.05 bar.20
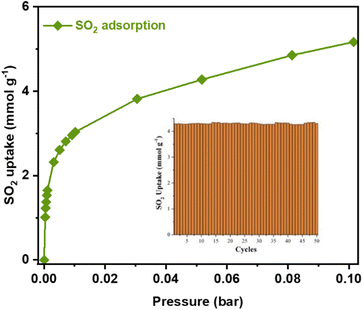
Interestingly, at a very low pressure of only 0.002 bar, Ni2(dobpdc) captures 1.98 mmol g−1 of SO2 (Fig. 2). In this case, only four MOF materials surpassed this value: Mg2(dobpdc) (2.35 mmol g−1),21 Ni-gallate (4.25 mmol g−1),22 Mg-gallate (6.09 mmol g−1),22 and Co-gallate (6.13 mmol g−1),22 at 298 K. Thus, this SO2 capture at such low pressure is highly relevant for detecting SO2.
Later, a structural stability test of Ni2(dobpdc) after the SO2 adsorption–desorption experiment was conducted. PXRD analysis confirmed the retention of the crystallinity (Fig. S5†) after the first SO2 sorption experiment. Moreover, an N2 adsorption isotherm at 77 K demonstrated that the porosity is not modified (BET area ≈ 3005 m2 g−1, Fig. S6†).
Moreover, the host–guest interaction (SO2–Ni2(dobpdc)) was quantified by calculating the isosteric heat of adsorption (ΔH) for SO2 at low coverage for a fully activated sample of Ni2(dobpdc) (two adsorption isotherms at 298 and 308 K were fitted to a Clausius–Clapeyron equation, Fig. S7†). Then, the calculated ΔH = −95.2 kJ mol−1 was demonstrated to be relatively high, suggesting a relatively strong interaction between SO2 and the walls of the MOF material. This ΔH value is also consistent with the hysteresis shown in Fig. 1. It is characteristic of SO2 and open metal site systems (e.g., Mg2(dobpdc),21 ΔH = −90.0 kJ mol−1). Also, it is observed a decrease in ΔH (Fig. S7†) when the SO2 loading is increased. Thus, open metal sites are first occupied by SO2 (higher energy binding sites), and then, at higher loadings, the SO2 molecule interacts through intermolecular forces with the pore walls of the MOF.
Therefore, cycling SO2 experiments at 298 K and 0.05 bar were further investigated in order to evaluate the stability of the SO2 adsorption–desorption performance and the regeneration capacity of Ni2(dobpdc) by simply applying vacuum (1.7 × 10−3 Torr) for 45 minutes and 298 K. Thus, it was shown that the SO2 capture capacity, at very low pressure, constantly continued for 50 adsorption–desorption cycles (4.31 ± 0.10 mmol g−1, Fig. 2, inset). This shows that SO2 is fully released during the subsequent desorption cycles. Also, PXRD analyses of the material after 50 adsorption/desorption cycles confirmed the retention of the crystal structure (Fig. S5†). At the same time, N2 adsorption isotherm at 77 K evidenced that the porosity was not modified (BET area ≈ 3005 m2 g−1, Fig. S6†). This result exhibits that SO2 can be fully released when SO2 cycle experiments are carried out without modifying the crystal structure of Ni2(dobpdc).
Up to this point, the SO2 capture by Ni2(dobpdc), particularly at very low pressure (0.002 bar), has been demonstrated. This is a fundamental requirement for SO2 detection. Ni2(dobpdc) also showed high SO2 cyclability and extraordinary chemical stability to SO2, desirable characteristics for SO2 detection.
Thus, another fundamental aspect of any detector is selectivity.23 A CO2 single-component adsorption isotherm was collected at 298 K and 1 bar (Fig. S9†). The SO2 adsorbed amount in Ni2(dobpdc) was higher than the CO2 in the whole pressure range. Then, we calculated the selectivity of SO2 over CO2 at different compositions (SO2/CO2) using the Python package (Table S1†), pyIAST,24 corroborating a remarkable SO2/CO2 selectivity by Ni2(dobpdc).
Once it was comprehensively established that the SO2 adsorption characteristics of Ni2(dobpdc) as a promising SO2 detector, the possibility of using this MOF material as a fluorescent SO2 detector was investigated, first, under UV light irradiation at λex = 350 nm (Fig. S12†), an activated sample of Ni2(dobpdc) showed a broad photoluminescence peak centred at λmax = 450 nm, as it is observed in Fig. 3. This is attributed to the dobpdc fragment as previously reported for Mg2(dobpdc).21 Then, another activated sample of Ni2(dobpdc) was exposed (saturated) to SO2 in our homemade in situ adsorption system (Fig. S8†) to measure its photoluminescence absorption properties of it later. Interestingly, the photoluminescence shifts to 405 nm and an increase of approximately 61 % in emission intensity was observed.
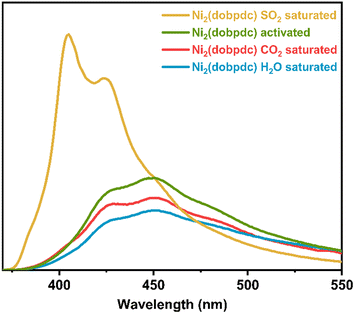 | ||
| Fig. 3 Solid-state emission spectra of activated Ni2(dobpdc) (green) and after exposure to SO2 (yellow), CO2 (red), and H2O (blue). | ||
These photoluminescence changes in position and intensity can be attributed to the electronic effect that SO2 applies on the framework when coordinated to the Ni-centres, which is successively transferred to the dobpdc ligand. A control experiment was conducted further to test the selectivity of Ni2(dobpdc). The fluorescence of Ni2(dobpdc) upon exposure to H2O and CO2 was measured (Fig. 3). Importantly, none of these molecules generated significant changes in the shape or intensity of the photoluminescence compared to the spectrum of activated Ni2(dobpdc).
Thus, since none of these conditions (exposure to H2O and CO2) significantly altered the shape or intensity of the emission, corroborated the selectivity of Ni2(dobpdc) to SO2, indicating that the change in fluorescence is exclusively due to SO2 adsorption-coordination and not due to other molecules. Later, five independent experiments were measured. An activated Ni2(dobpdc) sample was saturated with SO2. Consistently, it was found that the same absorption spectrum shifted to 405 nm, with an approximate 61 % increase in emission intensity.
Once it was demonstrated the SO2 detection selectivity for Ni2(dobpdc), over H2O and CO2, and the reproducibility of the detection of saturated samples Ni2(dobpdc) with SO2, the detection properties of this Ni(II)-based material at low SO2 pressure: non-saturated Ni2(dobpdc) with SO2 was investigated. Since Ni2(dobpdc) demonstrated a relatively high SO2 uptake at low pressure (P = 0.1 bar), vide supra, and this low pressure can be correlated to SO2 detection at low SO2 concentrations, it becomes crucial to investigate photoluminescence response at lower SO2 pressures (in this case, 0.1 bar).
Thus, an activated sample of Ni2(dobpdc) was exposed to 0.1 bar of SO2 (Fig. S8†). The emission spectrum was measured (Fig. 4). Interestingly, the broad photoluminescence peak remained centred at λmax = 450 nm. An increase in emission intensity of approximately 62 % was observed. Also, the reproducibility of this experiment was evaluated with 5 independent experiments (re-activation of the sample and re-exposure to 0.1 bar of SO2). It was found that an increase of approximately 62 % in emission intensity. It is worth mentioning that only the SO2 detection by Ni2(dobpdc) at 0.1 bar was performed. At this point, it cannot be translated to SO2 sensing. Although we consistently found an increase in the emission intensity when re-exposing to SO2 and re-activating the sample of Ni2(dobpdc) at 0.1 bar SO2. Nevertheless, it is not possible to quantify the precise amount of SO2 by photoluminescence. However, achieving a consistent and reproducible response is very promising when this Ni(II)-based MOF material is exposed to 0.1 or 1 bar of SO2.
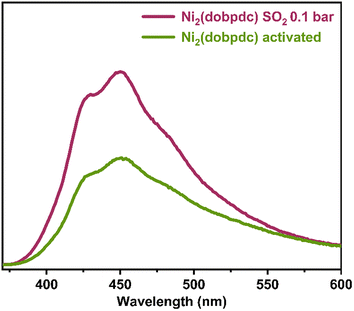 | ||
| Fig. 4 Comparison of solid-state emission spectra of Ni2(dobpdc) samples activated (green) and exposed to SO2 at 0.1 bar (pink). | ||
Finally, in order to investigate the plausible mechanism of SO2 detection by Ni2(dobpdc), a time-resolved photoluminescence (TRPL) experiment was performed using a 340 nm picosecond-pulsed LED as the excitation source (Fig. 5). TRPL experiments were carried out on an activated sample of Ni2(dobpdc) and an SO2-saturated sample. The photoluminescence decay was measured at three different emission wavelengths: 405 nm, which was the emission maximum of the SO2-saturated sample; 450 nm, corresponding to the emission maximum of the activated sample and 425 nm since this signal was observed in both spectra (Fig. S13–S16†). For all three emission wavelengths, it was observed that the average decay lifetimes increased upon SO2 exposure (Table S2†). Thus, the average fluorescence lifetime of the activated sample (at λemission = 405 nm) was 2.14 ns, while the lifetime of the SO2-saturated sample (at λemission = 405 nm) was 2.47 ns (Fig. 5). These results suggest that the coordination of SO2 molecules to the Ni(II) metal centres rigidify the molecular motions of the organic ligand (dobpdc). Subsequently, it hinders non-radiative decay pathways of the photoexcited state, causing the fluorescence lifetime to slow down. Thus, it increases the number of radiatively decaying excited species, leading to an improvement in the fluorescence intensity.25
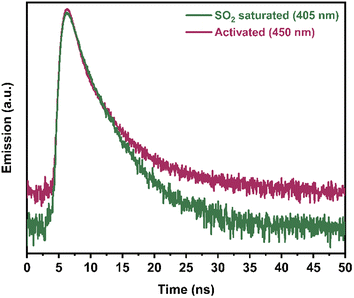 | ||
| Fig. 5 Time-resolved photoluminescence decay spectra of activated Ni2(dobpdc), and after exposure to SO2 measured at 405 nm at 340 nm excitation. | ||
One of the reasons why the wavelength shifts, is the change in energy due to the electronic transitions, where intermolecular interactions can considerably influence. It was previously reported that a strong interaction could considerably impact the excited state of some materials.26 Thus, this can indicate that the coordination of SO2 to all the Ni(II) centres is strong enough to increase the energy difference between the basal and excited states, causing the fully SO2-coordinated Ni2(dobpdc) to emit light at higher energies. In other words, a shift in the spectrum can be observed at higher energies, i.e., at shorter wavelengths.
To summarise, the SO2 adsorption and detection properties of a structurally stable MOF material entitled Ni2(dobpdc) were investigated. Ni2(dobpdc) demonstrated a high SO2 uptake at low pressure (4.3 mmol g−1 at 298 K and 0.05 bar), in combination with an excellent cyclability performance with a facile SO2 regeneration at room temperature. Fluorescence studies exhibited a significant change in the emission spectra after SO2 adsorption, with a clear SO2 detection selectivity, over H2O and CO2 and reproducible SO2 response when this Ni(II)-based MOF material was exposed to only 0.1 or 1 bar of SO2. Finally, time-resolved photoluminescence experiments suggested that the coordination of SO2 molecules to the Ni(II) metal centres rigidify the molecular motions of the organic ligand (dobpdc), leading to an increase in fluorescence intensity. Overall, this study postulates Ni2(dobpdc) as a promising candidate for SO2 detection.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
V. B. L.-C. and J. L. O. thank CONACYT for the Ph.D. fellowship (1005649, 1003953). The authors thank Dr. A. Tejeda-Cruz (powder X-ray; IIM-UNAM), PAPIIT UNAM (IN201123), México, for financial support. Thanks to U. Winnberg (Euro Health) for scientific discussions and G. Ibarra-Winnberg for conceptualising the design of this contribution. C. S. Hong also acknowledged financial support from the National Research Foundation of Korea (NRF-2021R1A2B5B03086313 and NRF-2019R1A6A1A11044070).References
- Z. Klimont, S. J. Smith and J. Cofala, Environ. Res. Lett., 2013, 8, 014003 CrossRef CAS.
- M. Matooane and R. Diab, Arch. Environ. Health, 2003, 58, 763–770 CrossRef CAS PubMed.
- P. Amoatey, H. Omidvarborna, M. S. Baawain and A. Al-Mamun, Process Saf. Environ. Prot., 2019, 123, 215–228 CrossRef CAS.
- J. Schwartz and D. W. Dockery, Am. Rev. Respir. Dis., 1992, 145, 600–604 CrossRef CAS PubMed.
- R. Reiss, E. L. Anderson, C. E. Cross, G. Hidy, D. Hoel, R. McClellan and S. Moolgavkar, Inhalation Toxicol., 2007, 19, 419–449 CrossRef CAS PubMed.
- F. C. Menz and H. M. Seip, Environ. Sci. Policy, 2004, 7, 253–265 CrossRef CAS.
- R. K. Srivastava, W. Jozewicz and C. Singer, Environ. Prog., 2001, 20, 219–228 CrossRef CAS.
- B. E. Alver, M. Sakizci and E. Yörükoğullari, Adsorpt. Sci. Technol., 2011, 29, 413–422 CrossRef CAS.
- B. Erdoğan Alver, J. Hazard. Mater., 2013, 262, 627–633 CrossRef PubMed.
- N. D. Hutson, B. A. Reisner, R. T. Yang and B. H. Toby, Chem. Mater., 2000, 12, 3020–3031 CrossRef CAS.
- A. J. Hernández-Maldonado, R. T. Yang, D. Chinn and C. L. Munson, Langmuir, 2003, 19, 2193–2200 CrossRef.
- W. Lu, Z. Wei, Z.-Y. Gu, T.-F. Liu, J. Park, J. Park, J. Tian, M. Zhang, Q. Zhang, T. Gentle III, M. Bosch and H.-C. Zhou, Chem. Soc. Rev., 2014, 43, 5561–5593 RSC.
- A. López-Olvera, S. Pioquinto-García, J. Antonio Zárate, G. Diaz, E. Martínez-Ahumada, J. L. Obeso, V. Martis, D. R. Williams, H. A. Lara-García, C. Leyva, C. V. Soares, G. Maurin, I. A. Ibarra and N. E. Dávila-Guzmán, Fuel, 2022, 322, 124213 CrossRef.
- A. López-Olvera, J. A. Zárate, E. Martínez-Ahumada, D. Fan, M. L. Díaz-Ramírez, P. A. Sáenz-Cavazos, V. Martis, D. R. Williams, E. Sánchez-González, G. Maurin and I. A. Ibarra, ACS Appl. Mater. Interfaces, 2021, 13, 39363–39370 CrossRef PubMed.
- J. L. Obeso, E. Martínez-Ahumada, A. López-Olvera, J. Ortiz-Landeros, H. A. Lara-García, J. Balmaseda, S. López-Morales, E. Sánchez-González, D. Solis-Ibarra, C. Leyva and I. A. Ibarra, ACS Appl. Energy Mater., 2020 DOI:10.1021/acsaem.2c02983.
- S. Yang, J. Sun, A. J. Ramirez-Cuesta, S. K. Callear, W. I. F. David, D. P. Anderson, R. Newby, A. J. Blake, J. E. Parker, C. C. Tang and M. Schröder, Nat. Chem., 2012, 4, 887–894 CrossRef CAS PubMed.
- T. M. McDonald, J. A. Mason, X. Kong, E. D. Bloch, D. Gygi, A. Dani, V. Crocellà, F. Giordanino, S. O. Odoh, W. S. Drisdell, B. Vlaisavljevich, A. L. Dzubak, R. Poloni, S. K. Schnell, N. Planas, K. Lee, T. Pascal, L. F. Wan, D. Prendergast, J. B. Neaton, B. Smit, J. B. Kortright, L. Gagliardi, S. Bordiga, J. A. Reimer and J. R. Long, Nature, 2015, 519, 303–308 CrossRef CAS PubMed.
- (a) D. Gygi, E. D. Bloch, J. A. Mason, M. R. Hudson, M. I. Gonzalez, R. L. Siegelman, T. A. Darwish, W. L. Queen, C. M. Brown and J. R. Long, Chem. Mater., 2016, 28, 1128–1138 CrossRef CAS; (b) T. M. McDonald, W. R. Lee, J. A. Mason, B. M. Wiers, C. S. Hong and J. R. Long, J. Am. Chem. Soc., 2012, 134(16), 7056–7065 CrossRef CAS PubMed.
- J. H. Carter, X. Han, F. Y. Moreau, I. da Silva, A. Nevin, H. G. W. Godfrey, C. C. Tang, S. Yang and M. Schröder, J. Am. Chem. Soc., 2018, 140(46), 15564–15567 CrossRef CAS PubMed.
- P. Brandt, S.-H. Xing, J. Liang, G. Kurt, A. Nuhnen, O. Weingart and C. Janiak, ACS Appl. Mater. Interfaces, 2021, 13, 29137–29149 CrossRef CAS PubMed.
- E. Martínez-Ahumada, D. won Kim, M. Wahiduzzaman, P. Carmona-Monroy, A. López-Olvera, D. R. Williams, V. Martis, H. A. Lara-García, S. López-Morales, D. Solis-Ibarra, G. Maurin, I. A. Ibarra and C. S. Hong, J. Mater. Chem. A, 2022, 10, 18636–18643 RSC.
- F. Chen, D. Lai, L. Guo, J. Wang, P. Zhang, K. Wu, Z. Zhang, Q. Yang, Y. Yang, B. Chen, Q. Ren and Z. Bao, J. Am. Chem. Soc., 2021, 143, 9040–9047 CrossRef CAS PubMed.
- K. Tan, S. Zuluaga, H. Wang, P. Canepa, K. Soliman, J. Cure, J. Li, T. Thonhauser and Y. J. Chabal, Chem. Mater., 2017, 29, 4227–4235 CrossRef CAS.
- C. M. Simon, B. Smit and M. Haranczyk, Comput. Phys. Commun., 2016, 200, 364–380 CrossRef CAS.
- L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105–1125 CrossRef CAS PubMed.
- A. P. Demchenko, Introduction to Fluorescence Sensing, Springer, Switzerland, 2015, ch. 3, pp. 69–126 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Material characterisation, and photoluminescence experiments. See DOI: https://doi.org/10.1039/d3nr02936k |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |