Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Metal–organic framework-derived trimetallic oxides with dual sensing functions for ethanol†
Xin-Yu
Huang
 a,
Ya-Ru
Kang
b,
Shu
Yan
a,
Ya-Ru
Kang
b,
Shu
Yan
 a,
Ahmed
Elmarakbi
a,
Ahmed
Elmarakbi
 c,
Yong-Qing
Fu
c,
Yong-Qing
Fu
 *c and
Wan-Feng
Xie
*c and
Wan-Feng
Xie
 *ad
*ad
aCollege of Electronics and Information, University-Industry Joint Center for Ocean Observation and Broadband Communication, Qingdao University, Qingdao 266071, P. R. China. E-mail: wfxie@qdu.edu.cn
bSchool of Integrated Circuits, University of Chinese Academy of Sciences, Beijing 100049, P. R. China
cFaculty of Engineering and Environment, Northumbria University, Newcastle upon Tyne NE1 8ST, UK. E-mail: richard.fu@northumbria.ac.uk
dDepartment of Physics, Dongguk University, Seoul 04620, South Korea
First published on 3rd April 2023
Abstract
Metal–organic framework (MOF)-derived metal oxide semiconductors have recently received extensive attention in gas sensing applications due to their high porosity and three-dimensional architecture. Still, challenges remain for MOF-derived materials, including low-cost and facile synthetic methods, rational nanostructure design, and superior gas-sensing performances. Herein, a series of Fe-MIL-88B-derived trimetallic FeCoNi oxides (FCN-MOS) with a mesoporous structure were synthesized by a one-step hydrothermal reaction followed by calcination. The FCN-MOS system consists of three main phases: α-Fe2O3 (n-type), CoFe2O4, and NiFe2O4 (p-type), and the nanostructure and pore size can be controlled by altering the content of α-Fe2O3, CoFe2O4, and NiFe2O4. The sensors based on FCN-MOS exhibit a high response of 71.9, a good selectivity towards 100 ppm ethanol at 250 °C, and long-term stability up to 60 days. Additionally, the FCN-MOS-based sensors show a p–n transition gas sensing behavior with the alteration of the Fe/Co/Ni ratio.
Introduction
Metal–organic frameworks (MOFs), a network with a three-dimensional porous structure and a large specific surface area, are constructed via coordination bonds between inorganic nodes and organic ligands.1–4 Compared to the most MOFs containing divalent metal cations, Fe-MOFs are more robust porous frameworks linked by trivalent metals.5 Among the well-known Fe3+-terephthalate MOFs, Fe-MIL-88B is a flexible framework with a three-dimensional hexagonal structure and exhibits continuous breathing during solvation/desolvation.6,7 Considering the outstanding nature of MOFs, recently, tremendous attention has been paid to the development of MOF-derived metal oxide semiconductors (MOSs).8,9 MOF-derived MOS materials exhibit multifunctional characteristics and have been utilized in various applications such as catalysis,10 oxygen evolution reactions,11,12 and ion batteries.13In the past few decades, various gas sensors have been developed for monitoring different gases. Among them, chemiresistive sensors are widely used for gas detection, owing to their portable and low-cost properties.14,15 Most chemiresistive sensors are MOS-based and operate at relatively high temperatures (typically around 300 °C) to achieve better sensing performances.16,17 However, MOFs cannot remain stable at such high temperatures because of the poor thermal stability of their organic skeleton. Besides, it is hard to effectively obtain the sensing signal of MOFs due to their poor electrical conductivity, and a few studies directly applied MOFs on chemiresistive sensors. To overcome the above limitation, it is feasible and promising to utilize MOF-derived MOSs as sensing materials, which can effectively optimize the nanostructure and increase the active sites of sensing materials, thus improving the gas-sensing performance further.
To date, many polymetallic oxides have been developed for gas sensing using bimetallic or trimetallic MOFs as templates. Introducing different metal ions into MOF-derived MOS systems can effectively increase their active sites and conductivity, leading to an improvement of the physicochemical reaction rates of target analytes.18,19 Accordingly, Fe-MIL-88B-derived polymetallic oxides are promising candidates to enhance gas sensing. Typically, the direct sacrifice of Fe-MIL-88B can obtain the monometallic oxide of α-Fe2O3, an n-type semiconductor with a bandgap (Eg) of 2.2 eV, which is often utilized for chemiresistive gas sensors.17 By doping the Fe-MIL-88B template with heteroatoms of similar periods, polymetallic oxides consisting of MFe2O4-based spinels can be formed. (M = Ni, Co, Zn, and Mn). Furthermore, MFe2O4-based spinels also show good gas-sensing performance.17 Among them, CoFe2O4 and NiFe2O4 spinel oxides, which are widely applied in chemiresistive gas sensors, are p-type semiconductor materials with bandgaps of 0.8 and 1.3 eV, respectively.20,21 For example, double-shelled nanocubes of Co3O4/CoFe2O4 show a response of 12.7 toward 10 ppm formaldehyde with a fast response/recovery speed (4/9 s) and an LOD of 300 ppb;22 hierarchically double-shelled hollow spheres of CoFe2O4 exhibit high sensitivity to ammonia gas at 240 °C;20 superfine and porous NiFe2O4 microspheres exhibit a high selectivity to acetone against other interfering gases, with a sensitivity of 27.4, an LOD of 200 ppb, and a fast response time of 2 s towards 100 ppm acetone;23 α-Fe2O3/NiFe2O4 nanotubes with a large specific surface area (118.03 m2 g−1) exhibit excellent sensing performance, including good sensitivity (23), a fast response speed (4 s), and long-term stability (30 days) towards 100 ppm acetone at 200 °C.24 Hence, it is worthwhile to develop α-Fe2O3, CoFe2O4, and NiFe2O4 nanocomposites as gas-sensing materials by using the Fe-MOF template doped with Co and Ni heteroatoms.
In this work, a series of Fe-MIL-88B-derived trimetallic FeCoNi metal oxide semiconductors (FCN-MOS) with a mesoporous nanostructure were successfully synthesized by a one-step hydrothermal reaction followed by calcination treatment. By optimizing the ratio of α-Fe2O3 and MFe2O4 in the FCN-MOS system, the nanostructure and pore size can be effectively tuned, resulting in the enhancement of gas-sensing performance. The FCN-MOS with the optimal molar ratio (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co
Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni = 7
Ni = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) has an elongated hexagonal rod-like structure with abundant mesopores and a large specific area. The sensor based on Fe7Co1.5Ni1.5 shows high sensitivity (S = 71.9), long-term stability, and good selectivity for ethanol at the optimal working temperature of 250 °C. Besides, an interesting p–n gas-sensing transition behavior was observed when varying the Fe/Co/Ni ratio, and the sensing mechanism was fully discussed.
1.5) has an elongated hexagonal rod-like structure with abundant mesopores and a large specific area. The sensor based on Fe7Co1.5Ni1.5 shows high sensitivity (S = 71.9), long-term stability, and good selectivity for ethanol at the optimal working temperature of 250 °C. Besides, an interesting p–n gas-sensing transition behavior was observed when varying the Fe/Co/Ni ratio, and the sensing mechanism was fully discussed.
Experimental
Synthesis of FCN-MOSs
All the chemicals were purchased from Aladdin Industrial Corporation and used without purification. These include ferric trichloride (FeCl3·6H2O), cobalt(II) acetate tetrahydrate (Co(Ac)2·4H2O), nickel(II) acetate tetrahydrate (Ni(Ac)2·4H2O), p-phthalic acid (1,4-BDC), N,N-dimethylformamide (DMF), acetone (C3H6O), and ammonia (NH3·H2O).A series of MOF-derived MOSs were synthesized using a hydrothermal method with the following calcination treatment. Generally, 1 mmol of FeCl3·6H2O was dissolved in 20 mL of a mixed solvent of DMF and acetone (with a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to form solution A. 1 mmol of 1,4-BDC was dissolved in 10 ml of a mixed solvent of DMF and acetone (volume ratio is 1
1) to form solution A. 1 mmol of 1,4-BDC was dissolved in 10 ml of a mixed solvent of DMF and acetone (volume ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to form solution B. Then, solution A was poured into solution B and magnetically stirred for 1 h at room temperature, while adding 100 μL of ammonia solution. The mixed solution was poured into a Teflon-lined stainless-steel autoclave (50 mL) and was heated to 120 °C for 24 h. The obtained products were washed with deionized water and absolute ethanol three times, followed by centrifugation, and then dried at 70 °C for 12 h in an oven. Finally, the samples were calcined in a muffle furnace at 500 °C for 3 h in an ambient environment. The calcined products were collected for analysis. Similarly, other types of FCN-MOSs were also prepared using the same procedures but with different Fe, Co, and Ni ratios. For convenience, these FCN-MOSs of Fe7Co1.5Ni1.5, Fe6Co2Ni2, Fe4Co4Ni2 and Fe2Co6Ni2 were named according to their molar ratios of 7
1) to form solution B. Then, solution A was poured into solution B and magnetically stirred for 1 h at room temperature, while adding 100 μL of ammonia solution. The mixed solution was poured into a Teflon-lined stainless-steel autoclave (50 mL) and was heated to 120 °C for 24 h. The obtained products were washed with deionized water and absolute ethanol three times, followed by centrifugation, and then dried at 70 °C for 12 h in an oven. Finally, the samples were calcined in a muffle furnace at 500 °C for 3 h in an ambient environment. The calcined products were collected for analysis. Similarly, other types of FCN-MOSs were also prepared using the same procedures but with different Fe, Co, and Ni ratios. For convenience, these FCN-MOSs of Fe7Co1.5Ni1.5, Fe6Co2Ni2, Fe4Co4Ni2 and Fe2Co6Ni2 were named according to their molar ratios of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, 6
1.5, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 4
2, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 of FeCl3·6H2O /Co(Ac)2·4H2O/Ni(Ac)2·4H2O, respectively.
2 of FeCl3·6H2O /Co(Ac)2·4H2O/Ni(Ac)2·4H2O, respectively.
Sensor fabrication and measurement
For sensing layers, first, FCN-MOSs (including Fe7Co1.5Ni1.5, Fe6Co2Ni2, Fe4Co4Ni2, and Fe2Co6Ni2) were grounded thoroughly in ethanol in an agate mortar to obtain a homogeneous paste, which was uniformly coated onto the surface of an alumina ceramic tube and dried at 70 °C under vacuum for 12 h to form a gas-sensing layer. Gas-sensing tests were performed using a WS-30A gas-sensing measurement system (Zhengzhou Winsen Electronic Technology Co., Ltd, China). During the tests, the target gases with the given concentrations were injected into the evaporation platform of a test chamber (18 L) via a micro syringe. The substrate platform was heated to evaporate the liquid target analyte, and then the targeted gas was evenly distributed in the test chamber after diffusion using an air circulation device. When the resistance reading of the sensor became stable, the test chamber was lifted open to introduce the ambient air. The response (S) of the gas sensor can be calculated from S = Ra/Rg (n-type) or S = Rg/Ra (p-type), where Ra and Rg represent the resistance values of the sensor in air and in the testing gases, respectively.Characterization
The surface morphology of the synthesized FCN-MOSs was characterized using a field emission scanning electron microscope (FE-SEM, Zeiss Gemini 500, Germany). Elemental mapping of the samples was performed using an energy-dispersive X-ray spectroscope (EDS, Oxford Link-ISIS 300, UK) operated at 15 kV. Crystal structures of the FCN-MOSs were studied using high-resolution transmission electron microscopy (HR-TEM, JEOL-2100 F, Japan), and interplanar spacings of lattice fringes were obtained using data analysis software (Gatan Digital Micrograph, USA). X-ray diffraction (XRD) was used to study the crystalline structure of the FCN-MOSs and was carried out using a Bruker D8 diffractometer equipped with Cu Kα radiation (λ = 0.154 nm). X-ray photoelectron spectroscopy (XPS) was used to determine the surface elemental composition and the chemical state of bonds of the FCN-MOSs, and an Escalab 250xi instrument (Thermo Scientific, USA) equipped with a monochromatic Al Kα source was used. The specific surface area and the pore size were determined by nitrogen (N2) adsorption–desorption measurement using Brunner–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) methods (Autosorb iQ Station 1, USA).Results and discussion
Characterization of FCN-MOSs
In this work, MOF-derived MOS materials were synthesized via a one-step hydrothermal method and after calcination treatment using the Fe-MIL-88B template. A series of FeCoNi-trimetallic oxides were obtained, and the overall design and implementation processes are systematically illustrated in Scheme 1.The morphologies of the Fe-MIL-88B template and its derivative were characterized by FE-SEM. The SEM images in Fig. S1† indicate that Fe-MIL-88B is a well-defined uniform hexagonal rod with pointed ends, which is consistent with previous reports.25,26 The SEM images of Fe7Co1.5Ni1.5 (Fig. 1a–c) show that the sample possesses an elongated hexagonal rod structure with an average length of 587 nm (Fig. S2†), similar to that of the MOF template. Furthermore, it can be observed that many nanoparticles are scattered on the sample surface and accumulate to form mesopores. For comparisons, the surface morphologies of Fe6Co2Ni2, Fe4Co4Ni2, and Fe2Co6Ni2 were also characterized (Fig. S3†). The results reveal that the Fe6Co2Ni2 and Fe4Co4Ni2 samples maintain an elongated hexagonal structure, and the nanoparticle sizes become larger as the Fe/Ni ratios decrease. However, for Fe2Co6Ni2, the structure became amorphous but still consisted of many nanoparticles.
TEM analysis was also performed to further investigate the morphology and structure. As shown in Fig. S4,† the Fe-MIL-88B template has a solid bulky rod-like structure with sharp edges. TEM images of Fe7Co1.5Ni1.5 (Fig. 1d and e) further confirm that the sample is composed of nanoparticles ranging in size from 6 to 18 nm, with an average size of approximately 11 nm (Fig. S5†). The HR-TEM image (Fig. 1f) reveals distinctive spacings of 0.368 and 0.220 nm corresponding to the (012) and (006) lattice planes of α-Fe2O3, and the (222) and (311) lattice planes of NiFe2O4 are 0.251 and 0.240 nm, respectively, whereas that of the (311) lattice plane of CoFe2O4 is 0.253 nm. The elements of Fe, Co, Ni, and O (Fig. 1h–k) are consistently distributed across the selected scanning area (Fig. 1g) from the EDS elemental mapping results, demonstrating that the acquired sample is constituted of Fe, Co, Ni, and O elements. The EDS spectrum (Fig. S6†) shows that Fe7Co1.5Ni1.5 contains 39.78% Fe, 1.52% Co and 15.47% Ni in atomic percentage, and the remaining concentration is oxygen.
It is vital to investigate the specific surface area and the pore size distribution of the MOF template and its derivative material. Hence, N2 adsorption–desorption measurements were carried out. Both samples show the H4 isotherm curves (Fig. S7†).27 The specific surface area of Fe-MIL-88B is calculated to be 55.70 m2 g−1 (Fig. S7a†) and has a high meso-microporosity (Fig. S7b†). The sample Fe7Co1.5Ni1.5 has a specific surface area of 39.51 m2 g−1 (Fig. S7c†) and a uniform distribution of main mesopores of about 8 nm (Fig. S7d†). The BET and BJH results demonstrate that using MOFs as a template to drive metal oxides can effectively obtain a relatively high specific surface area and abundant mesoporous.
The XRD pattern of the prepared Fe-MIL-88B is shown in Fig. S8,† for which the characteristic peaks correlate well to previous reports, demonstrating the successful synthesis of Fe-MIL-88B.7,25,26Fig. 2a indicates that the crystalline phases of α-Fe2O3, CoFe2O4, and NiFe2O4 are detected in Fe7Co1.5Ni1.5, Fe6Co2Ni2, and Fe4Co4Ni2, without the diffraction peaks of other phases. The observed diffraction peaks can be readily indexed to the crystal planes of α-Fe2O3 (PDF#33-0664), CoFe2O4 (PDF#03-0864), and NiFe2O4 (PDF#54-0964) phases, respectively, which agree well with the values from the powder diffraction file documents (Table S1†). The enlarged pattern in Fig. 2b shows that the intensity of the peak assigned to α-Fe2O3 (104) gradually decreases with the Fe/Ni ratio.
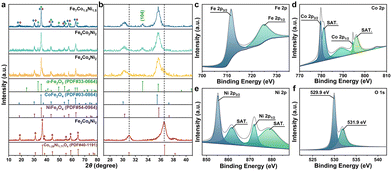 | ||
| Fig. 2 (a and b) XRD patterns of Fe7Co1.5Ni1.5, Fe6Co2Ni2, Fe4Co4Ni2, and Fe2Co6Ni2. High-resolution XPS spectra of Fe7Co1.5Ni1.5 for (c) Fe 2p, (d) Co 2p, (e) Ni 2p, and (f) O 1s. | ||
As it is well known, α-Fe2O3, an n-type sensing material, plays a critical role in enhancing the sensing performance. Therefore, the XRD results imply that the high α-Fe2O3 ratio of the samples (i.e., Fe7Co1.5Ni1.5 and Fe6Co2Ni2) might have excellent gas sensing properties. However, for the sample of Fe4Co4Ni2, as the α-Fe2O3 content is reduced with the decrease of Fe element proportion, the sensor based on Fe4Co4Ni2 might exhibit a transition from n-type to p-type sensing behavior accompanied by the deteriorated sensing performance. Nevertheless, it was found that the Fe2Co6Ni2 sample is composed of Co1.29Ni1.71O4 (PDF#40-1191) (Table S1†). The different crystalline structures of Fe2Co6Ni2 are attributed to the relatively low Fe ratio in the FCN-MOS, which results in the absence of the α-Fe2O3 phase and the formation of a Co1.29Ni1.71O4 phase. Therefore, the change in Fe2Co6Ni2 composition leads to morphology and structure conversion, further affecting its gas-sensing properties. XRD pattern of pristine α-Fe2O3 is shown in Fig. S9,† corresponding to the phase of α-Fe2O3 (PDF#33-0664).
The XPS survey spectrum of Fe7Co1.5Ni1.5 (Fig. 2c–f) shows the peaks of Fe 2p, Co 3d, Ni 3d, and O 1s. In the high-resolution spectrum of Fe 2p (Fig. 2c), two distinct peaks are observed at 711.6 eV for Fe 2p3/2 and 724.9 eV for Fe 2p1/2, corresponding to Fe3+ ions.28 The energy gap of 13.3 eV between these two peaks is close to the values reported in the standard spectrum of Fe 2p. In the Co 2p spectrum (Fig. 2d), there are two peaks located at 779.7 and 794.5 eV, which are associated with the valence states of Co+2 for Co 2p3/2 and Co 2p1/2, respectively.29 In the Ni 2p spectrum (Fig. 2e), two peaks at 855.2 and 871.9 eV correspond to Ni 2p3/2 and 2p1/2, respectively were observed. The Ni2+ and Ni3+ peaks have two satellite peaks of 2p3/2 and 2p1/2 at approximately 861.6 and 878.8 eV.30 The O 1s spectrum (Fig. 2f) contains two peaks centered at 529.9 and 531.9 eV, representing lattice oxygen and chemisorbed oxygen, respectively.31 The existence of chemisorbed oxygen is due to the surface chemisorbed O2 molecules, which is favorable for detecting the response of the target gas molecules.
The sensitivity of the FCN-MOS-based sensors substantially depends on the operating temperature. It is reported that conduction band electrons (e−) are dependent on the temperature, doping element, and volume mainly. The excited temperature (T) generates the electron concentration (n0) in the conductive band (Ec) for a semiconductor material, which is shown in eqn (1):32
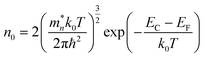 | (1) |
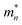 is the effective mass of the electron, k0 is Boltzmann's constant, h is Planck's constant, ħ = h/2π, and EF is the Fermi energy. The higher the temperature, the higher the concentration of electrons. However, for a gas sensor, the sensing performance is not linearly dependent on the temperature. Due to the weak adsorption energy of target gas molecules and oxygen species (O2−, O−, and O2−) on the surface of the sensing material at a high temperature, chemisorbed target gas molecules and oxygen species would easily escape from the surface of the sensing materials at a higher temperature, which could reduce the sensing catalytic reaction. As a result, it is essential and crucial to investigate the best operating temperature of the as-prepared sensors based on FCN-MOSs.
is the effective mass of the electron, k0 is Boltzmann's constant, h is Planck's constant, ħ = h/2π, and EF is the Fermi energy. The higher the temperature, the higher the concentration of electrons. However, for a gas sensor, the sensing performance is not linearly dependent on the temperature. Due to the weak adsorption energy of target gas molecules and oxygen species (O2−, O−, and O2−) on the surface of the sensing material at a high temperature, chemisorbed target gas molecules and oxygen species would easily escape from the surface of the sensing materials at a higher temperature, which could reduce the sensing catalytic reaction. As a result, it is essential and crucial to investigate the best operating temperature of the as-prepared sensors based on FCN-MOSs.
Gas-sensing performances of sensors based on Fe7Co1.5Ni1.5 and Fe6Co2Ni2
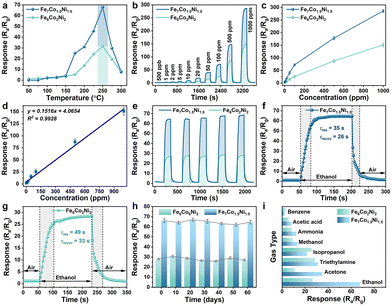
Fig. 3a shows the curves of sensing properties vs. detection temperature, and the sensitivity has no discernible differences at low temperatures (from 50 to 150 °C). Then, the sensitivity increases significantly after 150 °C, with a maximum sensing value occurring at 250 °C. The sensitivities of Fe7Co1.5Ni1.5 and Fe6Co2Ni2 show rapid decreases from 250 to 300 °C. Therefore, the optimal operating temperature for the Fe7Co1.5Ni1.5 and Fe6Co2Ni2-based sensors are set at 250 °C.Dynamic response–recovery curves of Fe7Co1.5Ni1.5 and Fe6Co2Ni2 in the ethanol concentration range from 0.5 to 1000 ppm are shown in Fig. 3b. Both gas sensors show good responses and recovery behaviors with various ethanol concentrations. With the increase of ethanol concentrations, the gas sensor responses exhibit a step-increasing pattern at the optimum working temperature of 250 °C. Compared to the Fe6Co2Ni2 based sensor, the response of Fe7Co1.5Ni1.5 is significantly increased with the concentration of targeted gases. These two sensors do not reach their saturation conditions when the ethanol concentration is 1000 ppm, as shown in Fig. 3c. In summary, the Fe7Co1.5Ni1.5 based sensor has a better response than Fe6Co2Ni2, owing to its optimal metal component ratio of Fe, Co, and Ni and the presence of the α-Fe2O3 phase. The LOD of the sensor to ethanol vapor was calculated utilizing a linear extrapolation of response sensitivity as a function of ethanol concentration (Fig. 3d). The calculated LOD result shows an ultra-low ethanol detection concentration of 30.7 ppb for Fe7Co1.5Ni1.5 operated at 250 °C. The repeatability and stability of ethanol sensing were further investigated using Fe7Co1.5Ni1.5 and Fe6Co2Ni2. Fig. 3e shows that after five cycles of exposure to 100 ppm of ethanol at 250 °C, both Fe7Co1.5Ni1.5 Fe6Co2Ni2 show repeatable curves, indicating their good stability. Fig. 3f and g show the response and recovery times of Fe7Co1.5Ni1.5 and Fe6Co2Ni2 exposed to 100 ppm ethanol at 250 °C. The Fe7Co1.5Ni1.5-based sensor has a response time (τres) of 35 s, which is faster than that (49 s) of Fe6Co2Ni2. Both sensors need a short time to return to 90% of their original resistance (e.g., Fe7Co1.5Ni1.5 needs 26 s and Fe6Co2Ni2 needs 33 s). Because the thermal energy is generally smaller than the activation energy for desorption, most of the chemical sensors do not show good reversibility, resulting in a prolonged recovery time (τrecov). The long-term stability of Fe7Co1.5Ni1.5 and Fe6Co2Ni2 was tested by exposing 100 ppm ethanol to the device once every ten days, measured at 250 °C for two months. Both the sensors show the preserved 98 percentage of their initial value with good stability after two months (Fig. 3h). Selectivity and cross-responses of these two sensors were investigated at 250 °C by exposing them to benzene (C6H6), acetic acid (C2H4O2), ammonia (NH3), methanol (CH4O), isopropanol (C3H8O), trimethylamine (C3H9N), acetone (C3H6O), and ethanol (C2H6O) (all with a fixed volume of 100 ppm). The obtained results are shown in Fig. 3i.
The above results clearly indicate that these two sensors are more sensitive to ethanol than other gases, particularly the Fe7Co1.5Ni1.5-based sensor. The phase of α-Fe2O3 can boost the redox process, whereas Fe7Co1.5Ni1.5 can be applied as a catalyst according to previous reports.24,33,34 Furthermore, the ethanol sensing properties of the sensor based on pristine α-Fe2O3 are shown in Fig. S10.† Fig. S10a† shows the response of α-Fe2O3 toward 100 ppm ethanol between 150 and 300 °C. The highest response (6.3) of ethanol gas was detected at 250 °C, indicating that the optimal operating temperature is 250 °C for α-Fe2O3, the same as the best working temperature of Fe7Co1.5Ni1.5. Fig. S10b† shows the dynamic response curves of α-Fe2O3 at 250 °C for different ethanol vapor concentrations ranging from 5 to 1000 ppm. The result indicates that the response values of α-Fe2O3 increase dramatically with increasing ethanol concentration, especially when the concentration is above 500 ppm. Fig. S10c and d† show the calibration curve of α-Fe2O3 at various concentrations. The slope at 0–70 ppm is quite small, with a low response, increasing between 70 and 100 ppm. Generally, the pristine α-Fe2O3 based sensor shows poor gas-sensing performances for ethanol. Therefore, the combination of α-Fe2O3, CoFe2O4, and NiFe2O4 provides greater catalytic selectivity for the redox interactions between chemisorbed oxygen and ethanol molecules in the sensing material.
Gas-sensing performances of sensors based on Fe2Co6Ni2 and Fe4Co4Ni2
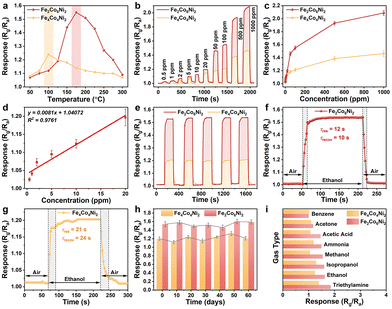
According to the aforementioned bipolar sensor design approach, the n-type sensing performance could be readily changed to p-type sensing performance by lowering the Fe element in the FCN-MOSs (e.g., Fe2Co6Ni2 and Fe4Co4Ni2).The response-temperature curves of Fe2Co6Ni2 and Fe4Co4Ni2 toward 100 ppm ethanol operated at temperatures ranging from 50 to 300 °C are shown in Fig. 4a. Obviously, both Fe2Co6Ni2 and Fe4Co4Ni2 showed a p-type sensing behavior at their optimum working temperatures of 100 °C and 175 °C. Still, their sensitivity was not as good as Fe7Co1.5Ni1.5 and Fe6Co2Ni2. The responses toward 100 ppm ethanol are 1.54 and 1.21 for these two sensors at their best operation temperatures of 100 °C and 175 °C, respectively. Because of a decrease in the Fe content, the amounts of p-type components in the CoFe2O4 and NiFe2O4 phases are increased. Therefore, sensors based on Fe2Co6Ni2 and Fe4Co4Ni2 exhibited a p-type sensing behavior.
Fig. 4b shows the dynamic response curves of Fe2Co6Ni2 and Fe4Co4Ni2 to ethanol with its concentration range of 0.5–1000 ppm. The response of Fe2Co6Ni2 is about 1.1 to 1.4 times larger than that of Fe4Co4Ni2. The response values as a function of ethanol concentration are shown in Fig. 4c, and they increase as the ethanol concentration increases. When the concentration is ∼20 ppm, the Fe2Co6Ni2 based sensor shows much larger response values. Because of the lower Fe ratio in the FCN-MOSs, Fe2Co6Ni2 shows a more significant response than Fe4Co4Ni2. A linear extrapolation was used to determine the LOD, and the results are shown in Fig. 4d. The obtained LOD for this sensor is 302.6 ppb. The repeatability of Fe2Co6Ni2 and Fe4Co4Ni2 samples’ responses to 100 ppm ethanol was also studied for 5 successive cycles, and the results are shown in Fig. 4e. For these two sensors, the obtained curves are nearly identical, with average response values of 1.54 and 1.20, respectively. The responses can be entirely returned to their starting levels in each cycle.
Fig. 4f and g compare the response/recovery times (τres/τrecov) of Fe2Co6Ni2 and Fe4Co4Ni2 toward 100 ppm ethanol at their optimum working temperatures (i.e., 175 °C for Fe2Co6Ni2 and 100 °C for Fe4Co4Ni2). The Fe2Co6Ni2-based sensor shows a faster response and recovery with its τres/τrecov value of 12/10 s, much shorter than that of Fe4Co4Ni2 (21/24 s). The Fe2Co6Ni2 and Fe4Co4Ni2-based sensors show much shorter τres/τrecov values than those of the Fe7Co1.5Ni1.5 and Fe6Co2Ni2-based sensors, which can be attributed to their relatively lower response values.
The long-term repeatability testing results of Fe2Co6Ni2 and Fe4Co4Ni2 toward 100 ppm ethanol at the optimal testing temperature of 175 °C are shown in Fig. 4h. The sensitivity has not been changed significantly within 60 days, proving the long-term repeatability of Fe2Co6Ni2 and Fe4Co4Ni2.
Fig. 4i presents the selectivity testing results of Fe2Co6Ni2 and Fe4Co4Ni2 when exposed to 100 ppm of various types of gases at their optimal working temperatures. The Fe2Co6Ni2-based sensor shows response values of 1.00, 1.17, 1.46, 1.49, 1.54, 1.60, 1.62, and 1.85 for benzene, acetone, acetic acid, ammonia, methanol, isopropanol, ethanol, and triethylamine gases, respectively. The results indicate that sensors based on Fe2Co6Ni2 and Fe4Co4Ni2 have a low selectivity towards ethanol gas.
Discussions on the gas-sensing mechanism
Table 1 compares ethanol sensing performances of various MOF-derived MOS-based sensors reported in the literature, including MOF-derived porous TiO2, MOF-derived CuO, MOF-derived Ga-doped Co3O4, zirconium-based MOFs, ZIF-67-derived Co3O4/NiCo2O4, and ZIF-8 MOF-derived ZnO. Based on Table 1, our newly developed FCN-MOS-based sensor can be operated at a relatively low working temperature and achieve a faster dynamic response with a higher sensitivity to ethanol. The main reasons are attributed to the novel MOF-derived nanostructures and the optimum proportion of the catalysts of α-Fe2O3, CoFe2O4, and NiFe2O4 phases, which can provide an effective gas diffusion path via a well-aligned porous structure.| Materials | Temperature (°C) | Concentration (ppm) | Response (S) | τ res/τrecov (s/s) |
|---|---|---|---|---|
| MOF-derived porous TiO235 | 250 | 500 | ∼46 | 74/102 |
| MOF-derived CuO36 | 275 | 100 | 12.1 | 102/40 |
| MOF-derived Ga-doped Co3O437 | 180 | 50 | ∼118 | 3/15 |
| Zirconium-based MOF38 | 150 | 100 | ∼1.4 | ∼50/400 |
| ZIF-67-derived Co3O4/NiCo2O439 | 180 | 100 | 26 | ∼4/∼6 |
| ZIF-8-derived ZnO40 | 300 | 1 | 6.7 | 1/28.5 |
| FCN-MOSs (this work) | 250 | 100 | 71.9 | 35/26 |
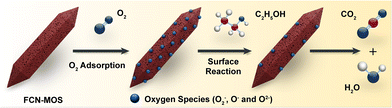
Fig. 5 schematically illustrates the adsorption of gas molecules, transfer of electrons, and surface reaction processes of FCN-MOS-derived oxide materials in air and ethanol gases, respectively. Generally, for the n-type sensing mechanism, when a sensing material is exposed to air, oxygen molecules are adsorbed and ionized. Thus, oxygen species such as O2−, O−, and O2− exist on the surface. During the oxygen ionization process, the electron concentration in a conductive band (Ec) reduces, then the resistance of the sensing material increases significantly. Therefore, the Ra value is increased. For a p-type sensor, electron extraction would produce a hole accumulation layer near the surface. Accordingly, the Ra value is decreased. Within the detection environment, with the gas such as ethanol in this study, depending on n- or p-types, the thickness of the electron (or hole) accumulation layer decreases (or increases) based on the reaction of CH3CH2OH (gas) + 8O− (ads) → 3CO2 + 3H2O + 8e−. The ethanol molecules transfer electrons to the n-type (α-Fe2O3) or p-type material (such as CoFe2O4 and NiFe2O4 phases), leading to a decrease/increase in electrical resistance, respectively.
According to the literature, p–n sensing mode transition is mainly related to the work function variations caused by the targeted gases.41 Kim et al. studied the p–n transition for CuO nanowires as a function of operating temperature for detecting the NO2 gas.42 In this study, the gas sensing transition from n-type to p-type is mainly due to the changes in the polarity of the sensing material, which means that the polarity of the sensing material can be changed from n-type to p-type when the phase composition of α-Fe2O3 (n-type) or CoFe2O4, and NiFe2O4 material (p-type) phases can be finely changed.
Conclusions
In summary, a series of Fe-MIL-88B-derived trimetallic FeCoNi oxides (FCN-MOS) were successfully synthesized using a one-step hydrothermal reaction with subsequent calcination treatment. The FCN-MOS system consists of α-Fe2O3, CoFe2O4, and NiFe2O4 and exhibits an elongated hexagonal rod-like structure with abundant mesopores. In addition, by altering the Fe, Co, and Ni ratio, the nanostructure and pore size of FCN-MOS can be effectively tuned, and a transition of gas-sensing behavior from n- to p-type can be achieved. The sensor based on FCN-MOS (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co
Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni = 7
Ni = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) exhibits excellent gas-sensing performance for ethanol, including high response (S = 71.9), long-term stability (over 60 days), and good selectivity, as well as a low detection limit of 500 ppb. The unique mesoporous structure and synergic effects of the α-Fe2O3, CoFe2O4, and NiFe2O4 phases are primarily responsible for the enhanced sensing performance. Overall, this work provides a facile route for synthesizing MOF-derived metal oxide semiconductors and proposes a novel material design strategy.
1.5) exhibits excellent gas-sensing performance for ethanol, including high response (S = 71.9), long-term stability (over 60 days), and good selectivity, as well as a low detection limit of 500 ppb. The unique mesoporous structure and synergic effects of the α-Fe2O3, CoFe2O4, and NiFe2O4 phases are primarily responsible for the enhanced sensing performance. Overall, this work provides a facile route for synthesizing MOF-derived metal oxide semiconductors and proposes a novel material design strategy.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC No. 41705098) and the International Exchange Grant (IEC/NSFC/201078) through the Royal Society and National Science Foundation of China (NSFC). This research was also supported by the Brain Pool program funded by the Ministry of Science and ICT through the National Research Foundation of Korea (No. 2021H1D3A2A01100019). The authors would like to thank Kehui Han from Shiyanjia Lab (www.shiyanjia.com) for the xrd analysis.References
- D. P. Erdosy, M. B. Wenny, J. Cho, C. DelRe, M. V. Walter, F. Jiménez-Ángeles, B. Qiao, R. Sanchez, Y. Peng, B. D. Polizzotti, M. O. de la Cruz and J. A. Mason, Nature, 2022, 608, 712–718 CrossRef CAS PubMed.
- S. Zhou, O. Shekhah, A. Ramírez, P. Lyu, E. Abou-Hamad, J. Jia, J. Li, P. M. Bhatt, Z. Huang, H. Jiang, T. Jin, G. Maurin, J. Gascon and M. Eddaoudi, Nature, 2022, 606, 706–712 CrossRef CAS PubMed.
- S. Wang, W. Xie, P. Wu, G. Lin, Y. Cui, J. Tao, G. Zeng, Y. Deng and H. Qiu, Nat. Commun., 2022, 13, 6673 CrossRef CAS PubMed.
- H. K. Lee, C. S. Koh, W.-S. Lo, Y. Liu, I. Y. Phang, H. Y. Sim, Y. H. Lee, G. C. Phan-Quang, X. Han, C.-K. Tsung and X. Y. Ling, J. Am. Chem. Soc., 2020, 142, 11521–11527 CrossRef CAS PubMed.
- T. Devic and C. Serre, Chem. Soc. Rev., 2014, 43, 6097–6115 RSC.
- C. Serre, C. Mellot-Draznieks, S. Surblé, N. Audebrand, Y. Filinchuk and G. Férey, Science, 2007, 315, 1828–1831 CrossRef CAS PubMed.
- P. Horcajada, F. Salles, S. Wuttke, T. Devic, D. Heurtaux, G. Maurin, A. Vimont, M. Daturi, O. David, E. Magnier, N. Stock, Y. Filinchuk, D. Popov, C. Riekel, G. Férey and C. Serre, J. Am. Chem. Soc., 2011, 133, 17839–17847 CrossRef CAS PubMed.
- W. Li, X. Guo, P. Geng, M. Du, Q. Jing, X. Chen, G. Zhang, H. Li, Q. Xu, P. Braunstein and H. Pang, Adv. Mater., 2021, 2105163 CrossRef CAS PubMed.
- M. Kim, R. Xin, J. Earnshaw, J. Tang, J. P. Hill, A. Ashok, A. K. Nanjundan, J. Kim, C. Young, Y. Sugahara, J. Na and Y. Yamauchi, Nat. Protoc., 2022, 17, 2990–3027 CrossRef CAS PubMed.
- V. Pascanu, G. González Miera, A. K. Inge and B. Martín-Matute, J. Am. Chem. Soc., 2019, 141, 7223–7234 CrossRef CAS PubMed.
- X. Wang, H. Xiao, A. Li, Z. Li, S. Liu, Q. Zhang, Y. Gong, L. Zheng, Y. Zhu, C. Chen, D. Wang, Q. Peng, L. Gu, X. Han, J. Li and Y. Li, J. Am. Chem. Soc., 2018, 140, 15336–15341 CrossRef CAS PubMed.
- Q. Qian, Y. Li, Y. Liu, L. Yu and G. Zhang, Adv. Mater., 2019, 31, 1901139 CrossRef PubMed.
- X. Xu, J. Liu, J. Liu, L. Ouyang, R. Hu, H. Wang, L. Yang and M. Zhu, Adv. Funct. Mater., 2018, 28, 1707573 CrossRef.
- W. Xie, Y. Ren, B. Yu, X. Yang, M. Gao, J. Ma, Y. Zou, P. Xu, X. Li and Y. Deng, Small, 2021, 17, 2103176 CrossRef CAS PubMed.
- L. Lüder, A. Gubicza, M. Stiefel, J. Overbeck, D. Beretta, A. Sadeghpour, A. Neels, P. N. Nirmalraj, R. M. Rossi, C. Toncelli and M. Calame, Adv. Electron. Mater., 2022, 8, 2100871 CrossRef.
- H. T. Jung, ACS Sens., 2022, 7, 912–913 CrossRef CAS PubMed.
- S. Y. Jeong, J. S. Kim and J. H. Lee, Adv. Mater., 2020, 32, e2002075 CrossRef PubMed.
- A. Bag, M. Kumar, D.-B. Moon, A. Hanif, M. J. Sultan, D. H. Yoon and N.-E. Lee, Sens. Actuators, B, 2021, 346, 130463 CrossRef CAS.
- S. Cao, Y. Xu, Z. Yu, P. Zhang, X. Xu, N. Sui, T. Zhou and T. Zhang, Small, 2022, 2203715 CrossRef CAS PubMed.
- L. Wang, Y. Wang, H. Tian, L. Qiao and Y. Zeng, Sens. Actuators, B, 2020, 314, 128085 CrossRef CAS.
- W. Remlalfaka, C. Murugesan, P. N. Anantharamaiah and N. Manikanda Prabu, Ceram. Int., 2021, 47, 11526–11535 CrossRef CAS.
- N. Zhang, S. Ruan, F. Qu, Y. Yin, X. Li, S. Wen, S. Adimi and J. Yin, Sens. Actuators, B, 2019, 298, 126887 CrossRef CAS.
- S. Zhang, W. Jiang, Y. Li, X. Yang, P. Sun, F. Liu, X. Yan, Y. Gao, X. Liang, J. Ma and G. Lu, Sens. Actuators, B, 2019, 291, 266–274 CrossRef CAS.
- T. Zhou, R. Zhang, Y. Wang and T. Zhang, Sens. Actuators, B, 2019, 281, 885–892 CrossRef CAS.
- G. Lee, S. Lee, S. Oh, D. Kim and M. Oh, J. Am. Chem. Soc., 2020, 142, 3042–3049 CrossRef CAS PubMed.
- D. Bara, E. G. Meekel, I. Pakamore, C. Wilson, S. Ling and R. S. Forgan, Mater. Horiz., 2021, 8, 3377–3386 RSC.
- X. Zhang, G. Li, Y. Zhang, D. Luo, A. Yu, X. Wang and Z. Chen, Nano Energy, 2021, 86, 106094 CrossRef CAS.
- Z. Dai, C.-S. Lee, Y. Tian, I.-D. Kim and J.-H. Lee, J. Mater. Chem. A, 2015, 3, 3372–3381 RSC.
- J. Chen, J. Zheng, Q. Huang, F. Wang and G. Ji, ACS Appl. Mater. Interfaces, 2021, 13, 36182–36189 CrossRef CAS PubMed.
- H. B. Zheng, H. H. Chen, Y. L. Wang, P. Z. Gao, X. P. Liu and E. V. Rebrov, ACS Appl. Mater. Interfaces, 2020, 12, 45987–45996 CrossRef CAS PubMed.
- X.-Y. Huang, Z.-T. Chi, W. Yang, Y. Deng and W.-F. Xie, Sens. Actuators, B, 2022, 361, 131715 CrossRef CAS.
- S. Sze and K. K. Ng, in Physics of Semiconductor Devices, 2006, pp. 5–75, DOI:10.1002/9780470068328.ch1.
- P. Wang, S. Z. Wang, Q. Han, D. Q. Zou, W. K. Zhao, X. D. Wang, C. Luo, X. Yang, X. Wu and W. F. Xie, Adv. Mater. Interfaces, 2020, 8, 2001831 CrossRef.
- D. Xie, F. Zhang, G. Dai, Z. Mao, K. Yu and F. Qu, New J. Chem., 2022, 46, 11368–11376 RSC.
- Y. Zhang, J. Zhang, G. Li, D. Leng, W. Wang, Y. Gao, J. Gao, Q. Liang, H. Lu and C. Wang, J. Mater. Sci.: Mater. Electron., 2019, 30, 17899–17906 CrossRef CAS.
- S. Wang, Z. Gao, G. Song, Y. Yu, W. He, L. Li, T. Wang, F. Fan, Y. Li, L. Zhang, X. Zhang, Y. Fu and W. Qi, J. Mater. Chem. C, 2020, 8, 9671–9677 RSC.
- H. Sun, X. Tang, J. Zhang, S. Lia and L. Liu, Sens. Actuators, B, 2021, 346, 130546 CrossRef CAS.
- J. H. Lee, T. T. T. Nguyen, L. H. T. Nguyen, T. B. Phan, S. S. Kim and T. L. H. Doan, J. Hazard. Mater., 2021, 403, 124104 CrossRef CAS PubMed.
- Y. Tao and W. Zeng, Ceram. Int., 2021, 47, 8441–8446 CrossRef CAS.
- Y. Xia, A. Pan, D. W. Gardner, S. Zhao, A. K. Davey, Z. Li, L. Zhao, C. Carraro and R. Maboudian, Sens. Actuators, B, 2021, 344, 130180 CrossRef CAS.
- A. Gurlo, N. Bârsan, A. Oprea, M. Sahm, T. Sahm and U. Weimar, Appl. Phys. Lett., 2004, 85, 2280–2282 CrossRef CAS.
- Y.-S. Kim, I.-S. Hwang, S.-J. Kim, C.-Y. Lee and J.-H. Lee, Sens. Actuators, B, 2008, 135, 298–303 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3nr00841j |
| This journal is © The Royal Society of Chemistry 2023 |