Synthesis of nickel carbonate hydroxide/zeolitic imidazolate framework-8 as a supercapacitors electrode
Yilong Gaoa,
Jianxiang Wua,
Wei Zhanga,
Yueyue Tana,
Jing Gaob,
Bohejin Tang*a and
Jiachang Zhaoa
aCollege of Chemistry and Chemical Engineering, Shanghai University of Engineering Science, Shanghai 201620, China. E-mail: tangbohejin@sues.edu.cn; Fax: +86-021-67791214; Tel: +86-021-67791214
bAnalysis and Determination Center, Changsha Research Institute of Mining and Metallurgy Limited Liability Company, Changsha 410012, China
First published on 12th August 2014
Abstract
The zeolitic imidazolate framework-8 (ZIF-8), nickel carbonate hydroxide (Ni2CO3(OH)2) and Ni2CO3(OH)2/ZIF-8 composite material are synthesized by a typical solvothermal method. In the Ni2CO3(OH)2/ZIF-8 material, the ZIF-8 acts as the host for the growth of Ni2CO3(OH)2. Their structure and surface morphology are characterized by X-ray diffraction, scanning electron microscopy, transmission electron microscopy and nitrogen adsorption–desorption isotherms. The porous structure combined with Ni2CO3(OH)2 maximizes the utilization of active material, resulting in a high specific capacitance. As electrode materials for supercapacitors, the ZIF-8, Ni2CO3(OH)2 and Ni2CO3(OH)2/ZIF-8 electrodes exhibit a specific capacitance of 140, 668 and 851 F g−1 respectively at a scan rate of 5 mV s−1 and good stability over 5000 cycles. In particular, Ni2CO3(OH)2/ZIF-8 is a promising candidate for the supercapacitor electrode.
The challenges of meeting the rapidly increasing demands for energy and developing a carbon-neutral economy require untiring efforts to exploit renewable and clean energy storage devices.1 Supercapacitors are considered as a promising candidate in the electrical energy storage field due to their high power density, long cycle life and safety tolerance to high rate charge and discharge.2 Energy storage mechanisms of supercapacitors are divided into two, double-layer capacitance arising from the charge separation at an electrode/electrolyte interface and pseudocapacitance arising from fast, reversible faradaic reactions occurring at or near a solid electrode surface.3 According to the energy storage mechanisms of double-layer capacitance and pseudocapacitance, the materials for supercapacitors should possess a high specific surface area to enhance the charge-storage capability.
Currently, metal–organic frameworks (MOFs) have gained particular attention in recent years as a novel class of nanoporous materials mainly because of their designable framework structures modularly built from transition-metal clusters as nodes and organic ligands as struts. As one of them, ZIF-8 has great properties such as large pore size (diameter of 11.6 Å), and large surface area (BET, 1413 m2 g−1), high thermal stability (up to 550 °C), and remarkable chemical resistance to boiling alkaline water and organic solvents.4 The applications of ZIF-8 in gas separation,5,6 catalysis,7,8 and sensing9 have been recently reported. However, there are a limited number of reports on MOFs as supercapacitor electrode materials up to now.10–12 In recent years, nickel hydroxyl compounds materials are typical active materials for electrochemical capacitors,13–17 which generate specific capacitance (SC) on Faradaic pseudo mechanism. Most of them display excellent electrochemical performance. For example, mesoporous α-Ni(OH)2 has a maximum specific capacitance of 1718 F g−1 at a scan rate of 5 mV s−1.18 Ni2CO3(OH)2, one of nickel hydroxyl compounds, is only as precursor to prepare nanostructured nickel compounds for supercapacitor electrode.19–22 In the light of most metal hydroxides having large theoretical SC values,17 we consider that Ni2CO3(OH)2 has a potential as electrode materials for supercapacitor.
In our previous study,23 ZIF-8 has been investigated as electrode materials for supercapacitor. Herein, Ni2CO3(OH)2 and Ni2CO3(OH)2/ZIF-8 are synthesized via a typical solvothermal method and investigated as potential electrode materials for supercapacitors with KOH electrolyte, respectively. The Ni2CO3(OH)2/ZIF-8 composite material shows excellent electrochemical properties with specific capacitance of 851 F g−1 at a scan rate of 5 mV s−1 and excellent cycling performance.
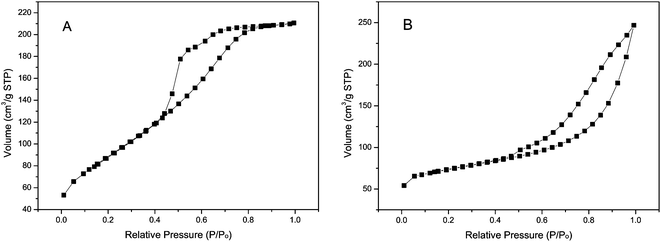
The crystal structure and purity of the samples were examined by powder XRD analysis in Fig. 1. A sharp peak at θ = 7.2° is observed on the XRD diffractogram of the ZIF-8, indicating that a highly crystalline material is achieved. The XRD pattern of the as synthesized ZIF-8 in this work is match up with the patterns from the single crystal data of pure ZIF-8.4 The difference of peaks intensity is due to the crystal preferred orientation. And the XRD pattern shown in Fig. 1(d) suggests that the obtained product is nickel carbonate hydroxide [Ni2CO3(OH)2] (JCPDS 35-0501). Fig. 1(c) is the XRD pattern of Ni2CO3(OH)2/ZIF-8, which shows that the Ni2CO3(OH)2 successfully loaded onto ZIF-8. Though the pattern of Ni2CO3(OH)2/ZIF-8 is a little different from the as synthesized ZIF-8, it is worth noting the crystalline order of the ZIF-8 host matrix mostly remains unchanged after loading Ni2CO3(OH)2, as shown by the comparison of the powder XRD patterns. No obvious diffractions are detected for Ni2CO3(OH)2 species from powder XRD patterns in Ni2CO3(OH)2/ZIF-8 samples, which may indicate Ni2CO3(OH)2 loadings are dispersed uniformly or too little in the samples. The morphology of the Ni2CO3(OH)2 and Ni2CO3(OH)2/ZIF-8 were examined by SEM and TEM. As shown in Fig. 2, the cotton-like structure with fibers as primary structures, shows loosely packed microstructure morphology characteristics. Nitrogen adsorption–desorption isotherms are shown in Fig. 3. The BET surface area of Ni2CO3(OH)2 and Ni2CO3(OH)2/ZIF-8 are 156.7 m2 g−1 and 313.8 m2 g−1, respectively.
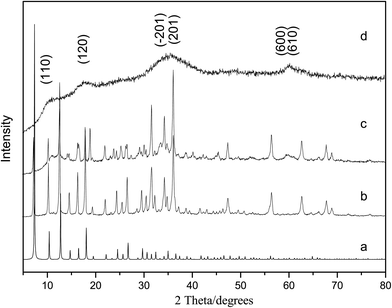 | ||
| Fig. 1 XRD patterns of (a) simulated ZIF-8; (b) as synthesized ZIF-8; (c) Ni2CO3(OH)2/ZIF-8; (d) Ni2CO3(OH)2. | ||
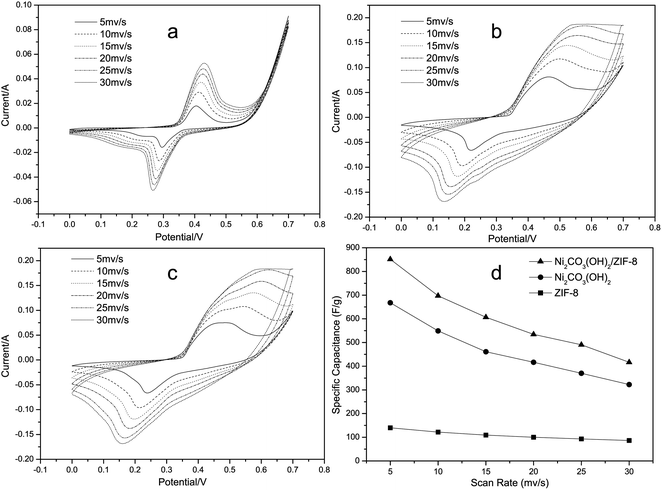
Cyclic voltammetry (CV) is considered as an important method in supercapacitor to evaluate the potential possibility of materials used for capacitive deionization and helps to obtain the electrochemical properties such as specific capacitance. Fig. 4(a–c) shows the CV curves of the active materials electrode in 6 M KOH electrolyte at various scan rates from 5 mV s−1 to 30 mV s−1. The CV shape of the electrode apparently reveals distinct pseudocapacitive characteristic, which is remarkably different from the closely ideal rectangular CV shape for an electric double-layer capacitor. Notably, a couple of redox peaks were observed within the potential range from 0 to 0.7 V, indicating that the electrochemical capacitance of the electrodes mainly results from the pseudocapacitance. The reactions of ZIF-8 electrodes seem to proceed through the following mechanism:24
| Zn(N2C4H6)2 + OH− ↔ Zn(N2C4H6)2OH + e | (1) |
The Ni2CO3(OH)2 electrode presents well-defined redox current peaks, and in line with the reversible reactions of Ni2+/Ni3+.17,25,26 Clearly, with increasing scan rate, the anodic peaks of the electrodes shift positively, while their cathodic peaks shift negatively. However, solution and electrode resistance distort slightly the current response at the switching potential and this distortion is dependent upon the scan rate,27 as can be seen from Fig. 4(b–c). The specific capacitances (SC) of electrodes are calculated according to the CV curves at different scan rates and the clear relationships are shown in Fig. 4(d). The SC value C can further be estimated according to the following equation:28
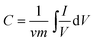 | (2) |
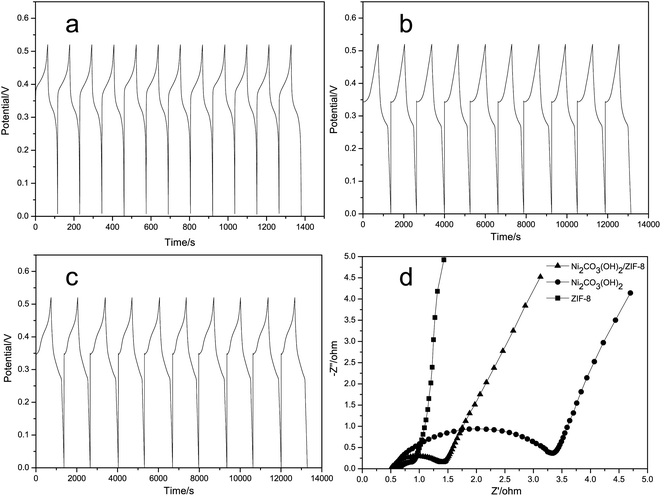
Chronopotentiometry (CP) curves for the ZIF-8, Ni2CO3(OH)2 and the Ni2CO3(OH)2/ZIF-8 electrodes are shown in Fig. 5(a–c) at a current density of 5 mA cm−2, respectively. The discharge curves of samples are not linear, suggesting the pseudocapacitive behavior of this electrode material, which is in agreement with CV analyses. The columbic efficiency is nearly 100% for each cycle of charge and discharge as can be seen from Fig. 5(a–c).
For further investigation of the actual electrochemical diffusion process, EIS spectra of the ZIF-8, Ni2CO3(OH)2 and the Ni2CO3(OH)2/ZIF-8 electrodes are measured in the frequency range from 0.01 to 105 Hz at open circuit potential with an ac excitation signal of 5 mV, as shown in Fig. 5(d). A single semicircle in the high frequency region is due to the internal resistance and capacitance. A linear inclination in the low frequency region is due to diffusion of electrolyte ions. The solution resistance Rs and the charge transfer resistance Rct can be obtained from the Nyquist plot, where the high frequency semicircle intercepts the real axis at Rs and (Rs + Rct), respectively. As shown in Fig. 5(d), the Rs are almost same, namely, 0.5 Ω, for Ni2CO3(OH)2 and the Ni2CO3(OH)2/ZIF-8 electrodes, and the Rs of the ZIF-8 electrode is 0.6 Ω. More importantly, the Rct of Ni2CO3(OH)2/ZIF-8 electrode is less than the Ni2CO3(OH)2 electrode's.
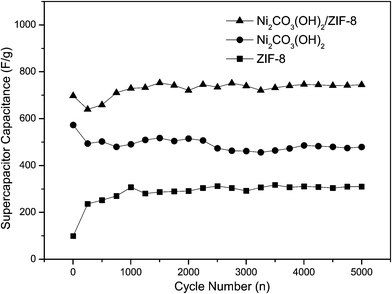
The specific capacitance as a function of cycle number is plotted in Fig. 6. It is observed that the specific capacitance of the ZIF-8 electrode continuously increases until about 700th cycle. The capacitance of Ni2CO3(OH)2 and Ni2CO3(OH)2/ZIF-8 electrode drop in this case are sharp at the beginning of 300 cycles and keeps slowly increasing till 2000 cycles, and then all the electrodes keep almost constant with slight fluctuations up to 5000 cycles. The capacitance decrease in the initial stage is possibly due to certain irreversible reaction in electrochemical process and increase in the following cycles is a full-activation at the electrode/electrolyte interface. The results show that the specific capacitances obtained from Ni2CO3(OH)2/ZIF-8 sample is superior to those derived from synthesized pure ZIF-8 and Ni2CO3(OH)2 materials.
Conclusion
In summary, we have demonstrated Ni2CO3(OH)2/ZIF-8 was successfully synthesized via a solvothermal method and as a supercapacitor electrode for the first time, respectively. XRD, SEM, TEM, nitrogen adsorption–desorption isotherms and electrochemical tests were employed to characterize the composite materials. The results show that the introduction of the ZIF-8 into Ni2CO3(OH)2 could enhance both specific capacitance and electrochemical stability of the Ni2CO3(OH)2/ZIF-8 composite electrodes. We expect that it bring light to new opportunities in the development of high performance energy storage devices by using rapidly growing MOFs as supports. Work is underway to expand ZIF-8 to other host MOFs for electrochemistry.Experimental section
Synthesis method of ZIF-8 (Zn(N2C4H6)2) was according to the literature of Yaghi et al.4 The process of Ni2CO3(OH)2/ZIF-8 complexes were the following: synthesized by mixing ZIF-8 and Ni(NO3)2·6H2O (mass ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) with 10 ml deionized water and the resulting mixtures were stirred at 293 K for about 2 h. Then, Na2CO3 solutions (0.3 mol L−1) were feeded into Teflon-lined and the molar ratio of Ni2+/CO32− can varied from 1
2) with 10 ml deionized water and the resulting mixtures were stirred at 293 K for about 2 h. Then, Na2CO3 solutions (0.3 mol L−1) were feeded into Teflon-lined and the molar ratio of Ni2+/CO32− can varied from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 to 1
1.1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3. When the addition was completed, the mixed solution adjusted to pH = 8.0–8.5 with ammonium hydroxide. The slurry was aged in dilute ammonia at 373 K in autoclave for at least 10 h. After being aged, the slurry was filtrated and dried. Then the dried precipitate was rewashed by hot water several times till the filtrate was almost clear when being detected by silver nitrate solution, and redried at 398 K in air flow. At last the dried green precipitate was grinded to get the finished product. As a control, Ni2CO3(OH)2 was also synthesized by the same method as described above, except that there was no ZIF-8 involved.
1.3. When the addition was completed, the mixed solution adjusted to pH = 8.0–8.5 with ammonium hydroxide. The slurry was aged in dilute ammonia at 373 K in autoclave for at least 10 h. After being aged, the slurry was filtrated and dried. Then the dried precipitate was rewashed by hot water several times till the filtrate was almost clear when being detected by silver nitrate solution, and redried at 398 K in air flow. At last the dried green precipitate was grinded to get the finished product. As a control, Ni2CO3(OH)2 was also synthesized by the same method as described above, except that there was no ZIF-8 involved.
The working electrode was prepared by mixing 75 wt% of the synthesized active material powder, 20 wt% of acetylene black and 5 wt% of poly(tetrafluoroethylene). After that, the resulting paste was immersed into a nickel foam (1 cm2) served as a current collector under a pressure of 10 MPa. The prepared electrode was dried at 90 °C oven for 2 h. The electrode of ZIF-8, Ni2CO3(OH)2 and Ni2CO3(OH)2/ZIF-8 contained about 3.75, 5.925 and 4.65 mg electroactive materials respectively and had a geometric surface area of 1 cm2.
Powder XRD data were collected on a Deutschland BRUKER D2 PHASER X-ray diffractometer with Cu Kα radiation. The 2θ was scanned in the range of 5–80° with a resolution of 0.02° s−1. Scanning electron microscopy (SEM) analysis (FEI INSPECT S50) was used to capture and determine the morphologies of the samples. The transmission electron microscopy (TEM) images were obtained on a Hitachi H-800 transmission electron microscope at an acceleration voltage of 200 kV. Nitrogen adsorption–desorption measurements for the products were performed using a Micromeritics ASAP 2460 instrument with a degassing temperature of 473 K, and using Barrett–Emmett–Teller (BET) calculations for the surface area. The pseudocapactive properties of the electrode were studied using cyclic voltammogram (CV), galvanostatic charge/discharge test and electrochemical impedance spectroscopy (EIS) in 6 M KOH aqueous solution. CVs and galvanostatic charge/discharge tests were conducted using the CHI660D electrochemical analyzer using a three-electrode configuration with the active material powder electrode as working electrode, platinum sheet as counter electrode and Ag as reference electrode.12 The EIS measurements were conducted for the working electrode in a frequency range of 100 kHz to 0.01 Hz with ac perturbation of 5 mV.
Acknowledgements
This work was supported by the Fund of Graduate Innovation Project, College of Chemistry and Chemical Engineering, Shanghai University of Engineering Science (A-0903-13-01078).References
- N. S. Lewis and D. G. Nocera, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 15729–15735 CrossRef CAS PubMed.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- B. E. Conway, J. Electrochem. Soc., 1991, 138, 1539–1548 CrossRef CAS PubMed.
- K. S. Park, Z. Ni, A. P. Cote, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O'Keeffe and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186–10191 CrossRef CAS PubMed.
- H. Bux, F. Liang, Y. Li, J. Cravillon, M. Wiebcke and J. Caro, J. Am. Chem. Soc., 2009, 131, 16000–16001 CrossRef CAS PubMed.
- S. R. Venna and M. A. Carreon, J. Am. Chem. Soc., 2009, 132, 76–78 CrossRef PubMed.
- H.-L. Jiang, B. Liu, T. Akita, M. Haruta, H. Sakurai and Q. Xu, J. Am. Chem. Soc., 2009, 131, 11302–11303 CrossRef CAS PubMed.
- C. Chizallet, S. Lazare, D. Bazer-Bachi, F. Bonnier, V. Lecocq, E. Soyer, A.-A. Quoineaud and N. Bats, J. Am. Chem. Soc., 2010, 132, 12365–12377 CrossRef CAS PubMed.
- G. Lu and J. T. Hupp, J. Am. Chem. Soc., 2010, 132, 7832–7833 Search PubMed.
- D. Y. Lee, S. J. Yoon, N. K. Shrestha, S.-H. Lee, H. Ahn and S.-H. Han, Microporous Mesoporous Mater., 2012, 153, 163–165 CrossRef CAS PubMed.
- D. Y. Lee, D. V. Shinde, E.-K. Kim, W. Lee, I.-W. Oh, N. K. Shrestha, J. K. Lee and S.-H. Han, Microporous Mesoporous Mater., 2013, 171, 53–57 CrossRef CAS PubMed.
- R. Díaz, M. G. Orcajo, J. A. Botas, G. Calleja and J. Palma, Mater. Lett., 2012, 68, 126–128 CrossRef PubMed.
- C.-C. Hu, J.-C. Chen and K.-H. Chang, J. Power Sources, 2013, 221, 128–133 CrossRef CAS PubMed.
- D.-L. Fang, Z.-D. Chen, X. Liu, Z.-F. Wu and C.-H. Zheng, Electrochim. Acta, 2012, 81, 321–329 CrossRef CAS PubMed.
- L. Cao, L.-B. Kong, Y.-Y. Liang and H.-L. Li, Chem. Commun., 2004, 1646–1647 RSC.
- S. Yang, X. Wu, C. Chen, H. Dong, W. Hu and X. Wang, Chem. Commun., 2012, 48, 2773–2775 RSC.
- L.-B. Kong, L. Deng, X.-M. Li, M.-C. Liu, Y.-C. Luo and L. Kang, Mater. Res. Bull., 2012, 47, 1641–1647 CrossRef CAS PubMed.
- S. Xing, Q. Wang, Z. Ma, Y. Wu and Y. Gao, Mater. Lett., 2012, 78, 99–101 CrossRef CAS PubMed.
- Y. Gu, Z. Lu, Z. Chang, J. Liu, X. Lei, Y. Li and X. Sun, J. Mater. Chem. A, 2013, 1, 10655–10661 CAS.
- G. Zhang, L. Yu, H. E. Hoster and X. W. Lou, Nanoscale, 2013, 5, 877–881 RSC.
- J. Zhu, J. Jiang, Y. Feng, G. Meng, H. Ding and X. Huang, ACS Appl. Mater. Interfaces, 2013, 5, 2634–2640 CAS.
- J. Zhu, J. Jiang, J. Liu, R. Ding, H. Ding, Y. Feng, G. Wei and X. Huang, J. Solid State Chem., 2011, 184, 578–583 CrossRef CAS PubMed.
- Y. Gao, J. Wu, W. Zhang, Y. Tan, J. Zhao and B. Tang, Mater. Lett., 2014, 128, 208–211 CrossRef CAS PubMed.
- S. Horike, D. Umeyama and S. Kitagawa, Acc. Chem. Res., 2013, 46, 2376–2384 CrossRef CAS PubMed.
- U. M. Patil, K. V. Gurav, V. J. Fulari, C. D. Lokhande and O. S. Joo, J. Power Sources, 2009, 188, 338–342 CrossRef CAS PubMed.
- J. Liu, C. Cheng, W. Zhou, H. Li and H. J. Fan, Chem. Commun., 2011, 47, 3436–3438 RSC.
- M. Aghazadeh, A. N. Golikand and M. Ghaemi, Int. J. Hydrogen Energy, 2011, 36, 8674–8679 CrossRef CAS PubMed.
- N. A. M. Barakat, A. G. El-Deen, G. Shin, M. Park and H. Y. Kim, Mater. Lett., 2013, 99, 168–171 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |