Parallel nanoliter detection of cancer markers using polymer microchips
Anja
Gulliksen
*ab,
Lars
Anders Solli
c,
Klaus
Stefan Drese
d,
Olaf
Sörensen
d,
Frank
Karlsen
ae,
Henrik
Rogne
f,
Eivind
Hovig
g and
Reidun
Sirevåg
b
aNorChip AS, Industriveien 8, 3490 Klokkarstua, Norway. E-mail: anja.gulliksen@norchip.com; Fax: +47 32 79 88 01; Tel: +47 40 40 34 88
bUniversity of Oslo, Dept. of Molecular Biosciences, 0316 Oslo, Norway
cNTNU, Dept. of Energy and Process Engineering, 7491 Trondheim, Norway
dIMM, Fluidik und Simulation, 55129 Mainz, Germany
eBuskerud University College, Kongsgata 51, 3019 Drammen, Norway
fSINTEF ICT, Dept. of Microsystems & Nanotechnology, 0314 Oslo, Norway
gNorwegian Radium Hospital, Dept. of Tumor Biology, Montebello, 0310 Oslo, Norway
First published on 28th January 2005
Abstract
A general multipurpose microchip technology platform for point-of-care diagnostics has been developed. Real-time nucleic acid sequence-based amplification (NASBA) for detection of artificial human papilloma virus (HPV) 16 sequences and SiHa cell line samples was successfully performed in cyclic olefin copolymer (COC) microchips, incorporating supply channels and parallel reaction channels. Samples were distributed into 10 parallel reaction channels, and signals were simultaneously detected in 80 nl volumes. With a custom-made optical detection unit, the system reached a sensitivity limit of 10−6 µM for artificial HPV 16 sequences, and 20 cells µl−1 for the SiHa cell line. This is comparable to the detection limit of conventional readers, and clinical testing of biological samples in polymer microchips using NASBA is therefore possible.
Introduction
Several studies have demonstrated that the presence of the human papilloma virus (HPV) is a prerequisite for the development of cervical cancer, the second most common cancer in women.1,2 Screening of cervical cancer is mainly done by cytological testing. However, this method has both poor reproducibility and specificity, as well as limited sensitivity.3 Therefore, new diagnostic methods have been developed. The present on-line technology identifies high-risk HPV mRNA transcripts employing real-time nucleic acid sequence-based amplification (NASBA).4–7 Briefly, NASBA is an isothermal (41 °C) method specifically designed for amplification of any single-stranded RNA and DNA sequence, by using three different enzymes simultaneously. The use of a specific and sensitive technology, such as NASBA, for detection of high-risk HPV types, makes it possible to meet the increasing demands for diagnostic precision and prognostic information, and to prevent incorrect diagnosis based on subjective decisions.We present experimental evidence of real-time NASBA detection in cyclic olefin copolymer (COC) microchips, with 80 nl detection volumes. The sample is automatically distributed into 10 parallel reaction channels for simultaneous detection, making it possible to specifically amplify and detect several different targets with high sensitivity on just one sample. The work presented here is part of a project towards a fully automated and disposable diagnostic microsystem with integrated sample preparation and detection modules for virus and bacteria identification. Shorter handling time, combined with reduced reagent and sample consumption, will be benefits of this system compared to conventional methods (Fig. 1).
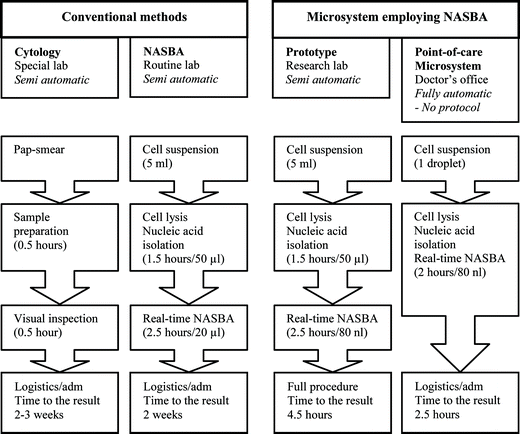 | ||
| Fig. 1 Comparison of conventional methods and a point-of-care microsystem for detection of cervical cancer. The false negative results for cytology is 69% for the first time tests. Employing NASBA, the false negative result is reduced to 23%. The time scale is approximate, since the analysis can be performed using different methods and instruments. | ||
Materials and methods
Microchip fabrication
Microchips, incorporating supply channels, reaction channels and microfluidic actuation systems, were injection molded in COC polymer.8 A photograph of the microchip is shown in Fig. 2(a). An actuation system was implemented on the microchip for liquid plug movement. However, the actuation mechanism is not described here, as it was not applied to these experiments. A description of the design and actuation functions of the chips is presented elsewhere.9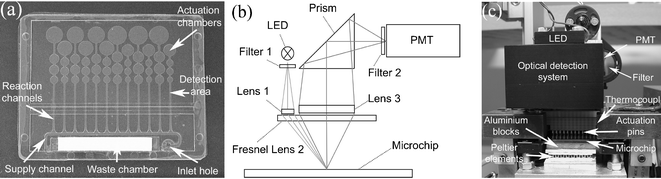 | ||
| Fig. 2 (a) A photograph of the COC microchip with dimensions of 50 × 40 mm. (b) Sketch of the optical geometry. (c) Photograph of the major components in the instrument. | ||
The microchips were oxygen plasma activated prior to coating with 5% (w/v) polyethylene glycol (PEG) in methanol (Sigma Chemical Co, St. Louis, MO). A cotton linter filter (Schleicher & Schuell BioScience GmbH, Relliehausen, Germany) was placed in the waste chamber, and the chips were sealed by welding a 75 µm COC membrane to the substrate. To block out background fluorescence from the thermal pads in the instrument, gold (25 nm) was sputtered on the back of the chip.
Optical detection system
The optical detection system was redesigned from an earlier prototype,10 to increase light intensity and reduce component costs (Fig. 2(b)). Light emitting diodes (LED) (Lumileds, San Jose, CA), with 130 mW centred at 470 nm, excited the fluorophores from above at an angle of 26° to the plane of the chip surface. Hence, the excitation light is reflected away from the light path of the detection unit. Scattered excitation light entering the detection unit was reduced by a bandpass filter (Chroma Technologies Corp, Brattleboro, VT), 465 nm–500 nm, and collimated through a lens (Melles Griot, Santa Clara, CA), ∅12.5 mm/f30 mm. An off-axis fresnel lens, ∅50 mm/f25 mm, focused the collimated light onto the reaction chambers. Fluorophores, excited at 494 nm and emitting at 525 nm, were activated and the light was collected in the center of the fresnel lens, passed through a lens, ∅25 mm/f100 mm, a prism (Melles Griot), a bandwidth filter, 500 nm–545 nm, and an aperture, before being detected by a photomultiplier-tube (PMT) (Hamamatsu, Shizuoka, Japan). A 2 × 2 mm2 area of the reaction channel was illuminated by the LED, corresponding to a detection volume of 80 nl (400 × 2000 × 100 µm3).Sequential measurements of the reaction chambers were performed by automatically moving the chip underneath the optical unit. Each channel was measured for 1 s on each scanning cycle, using a digital lock-in system operating at 1 kHz. A complete chip cycle took 90 s. Data were collected and processed using MATLAB (The MathWorks Inc., Natick, MA). Fig. 2(c) shows a photograph of the instrument set-up.
Instruments
Peltier elements (Marlow Industries Inc., Dallas, TX) with aluminum blocks mounted on top formed the chip holder. A thermal pad was placed on the blocks for thermal contact to the chip. A thermocouple was integrated into the chip holder, with feedback to the Peltier elements. Temperature regulation was controlled externally by the use of MATLAB. The system was calibrated with a commercial temperature calibration instrument (Fluke, Everett, WA) and platinum resistance sensors, both with an accuracy of ±0.1 °C. Temperature calibrations were performed both on the aluminum block, and on top of a dummy microchip, without a membrane. The overall temperature accuracy of the system was within ±1 °C.The instrument was equipped with a movable chip holder for alignment of the polymer microchip, automatic actuation and optical positioning. The servomotors (Omron Electronics, Kyoto, Japan) were regulated by a physical signaling sublayer (PLS) (Saia-Burgess Electronics AG, Murten, Switzerland), programmed with PG 5 (Saia-Burgess Electronics AG). All communications were run through a serial line (RS232) and controlled by MATLAB.
Results from the two outermost channels on the microchip were excluded in this work, because of a design fault in the instrument.
For comparison, sample solutions were also tested using microplates in a conventional microplate reader, Lambda FL600 (Bio-Tek Instruments, Winooski, VT). The total detection volume of the Lambda FL600 was 20 µl.
Sample material
The SiHa cell line, with 1–2 copies of integrated HPV 16 DNA per cell, was used as a model system.11–13 The cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and maintained in Dulbecco's modified Eagles medium (DMEM), supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine and 25 µg m−1 gentamycin. The cells were incubated at 37 °C in a 5% CO2 atmosphere, trypsinated, counted in a Bürker chamber, and lysed in lysis buffer (bioMérieux, Boxtel, the Netherlands), before the nucleic acids were isolated and extracted using a NucliSens Extractor (bioMérieux).14In addition, artificial HPV 16 sequences, from the PreTect® HPV-Proofer kit (NorChip AS, Klokkarstua, Norway) were used as targets in the reaction. To define the lower detection limit of the system, dilution series were tested. Serial dilutions ranging from 10−10 µM to 10−1 µM were tested using the artificial HPV 16 sequence, whereas the SiHa cell line was tested over a range from 2 × 10−2 cells µl−1 to 2 × 103 cells µl−1. SiHa cell line samples (250 cells µl−1) have been previously used as positive controls for detection of HPV 16 in biopsies from cervical cones6,17 in conventional microplates.
NASBA procedures
All reagents required to perform the NASBA amplification and the HPV detection were supplied as part of the PreTect® HPV-Proofer kit. The final concentration of the reaction mixture: 40 mM Tris-HCl (pH 8.5), 70 mM KCl, 12 mM MgCl2, 5 mM DTT, 1 mM dNTP, 2 mM ATP, 2 mM CTP, 2 mM UTP, 1.5 mM GTP, 0.5 mM ITP, 0.2 µM of each primer, 0.4 µM molecular beacon probe (FAM/Dabsyl), 375 mM sorbitol, 0.119 g l−1 BSA, 15% (v/v) DMSO, 6.4 U AMV RT, 32 U T7 RNA polymerase and 0.08 U RNase H.The reagent mixture except for the enzymes (26 µl) and sample material (13 µl) were mixed manually and heated on a conventional block heater at 65 °C for 2 min. The mixture was subsequently incubated at 41 °C for 2 min, after which the enzymes (13 µl) were added. This mixture was then immediately applied to the microchip and distributed into 10 parallel reaction channels. To ensure that all individual reaction channels were filled, one actuation chamber on each channel was punctured before the addition of the mixture to the microchip. This caused the reaction channels to be filled due to capillary forces. Excess reaction mixture was drawn into the waste chamber and absorbed by the filter, completely separating the fluids in individual reaction channels. Chip movement, process control and measurements were handled by the instrument.
For comparison, the ten-fold serial dilutions of the artificial HPV 16 sequence and of the SiHa cell line were tested, both in the microchip and in the conventional system. Reaction mixtures were prepared in the same way for both systems. The reaction volume for the conventional system was 20 µl.
For negative controls, water for molecular biology (DNase or RNase not detected, Sigma Chemical Co.) was added instead of sample material. All experiments were run for 2.5 h at 41 °C.
Calculations
Experimental results were processed with a dedicated NASBA regression calculation program, PreTect Data Analyzer (NorChip AS), based on polynomial regression algorithms. The final fluorescence level was divided with the initial fluorescence level and all reactions with a ratio larger than 1.7 were considered positive. Time-to-positivity (TTP)15,16 was chosen as the point of onset for exponential increase. The average slopes were calculated from the data between 10 and 80% increase in initial fluorescence level. The detection limit of the microchips was defined as the lowest concentration tested where all 10 reaction channels were positive.Results and discussion
Identification of the HPV 16 sequence and the SiHa cell line utilizing real-time NASBA was successfully performed in polymer microchips with a detection volume of 80 nl. Fig. 3 shows the result obtained from one microchip experiment using a SiHa cell line and a HPV 16 sequence. The graphs are clearly positive, and reveal the same sigmoid curvature as when samples were tested using regular 20 µl volumes and conventional readers.5,10,16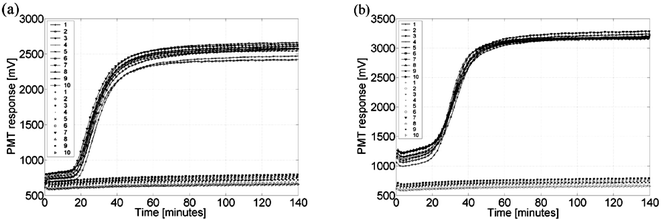 | ||
| Fig. 3 A (a) SiHa cell line sample (2000 cells µl−1) and a (b) HPV 16 sequence sample (0.1 µM) tested on a microchip. Solid lines characterize positive amplification reactions while no lines represent negative controls. The key numbers indicate the reaction channels on the microchip from left to right. | ||
To characterize the amplification reactions, several different parameters were evaluated: the fluorescence ratio, time-to-positivity (TTP), the average slope of the linear part of the curve, the number of positive amplifications, the number of polymer microchips and microplate reactions tested. The values shown in Table 1 represent the average values and the standard deviations of all the positive samples tested within the different dilution series. In comparison, the standard deviations of: the fluorescent ratio, the TTP and the average slope for an individual microchip ranged from (0.1–0.5), (0.0–14.5) and (1.2–19.6), respectively. For most experiments, the standard deviations, between parallel reaction channels on one microchip, are in the lower part of the range.
| Concentration | Ratio | TTP/min | Average slope: Microchips /mV min−1; Conventional/Fluorescence units min−1 | No. of positive reactions/Total no. of reactions | No. of positive reactions/Total no. of reactions | |||
|---|---|---|---|---|---|---|---|---|
| HPV 16 sequence /µM | 80 nl Microchips | 20 µl Conventional | 80 nl Microchips | 20 µl Conventional | 80 nl Microchips | 20 µl Conventional | 80 nl Microchips | 20 µl Conventional |
| 10−1 | 2.8 ± 0.3 | 6.5 ± 0.2 | 13.9 ± 4.6 | 14.0 ± 0.8 | 45.6 ± 10.4 | 111.2 ± 19.3 | 40 / 40 | 6 / 6 |
| 10−2 | 3.1 ± 0.4 | 6.7 ± 0.3 | 14.7 ± 4.0 | 11.8 ± 1.5 | 43.5 ± 9.5 | 96.3 ± 28.3 | 40 / 40 | 6 / 6 |
| 10−3 | 2.7 ± 0.4 | 6.5 ± 0.3 | 9.0 ± 2.1 | 15.3 ± 1.8 | 46.0 ± 17.7 | 113.1 ± 33.6 | 30 / 30 | 6 / 6 |
| 10−4 | 2.8 ± 0.3 | 5.2 ± 1.1 | 22.2 ± 4.5 | 23.8 ± 4.7 | 35.1 ± 17.9 | 94.4 ± 58.9 | 30 / 30 | 6 / 6 |
| 10−5 | 2.6 ± 0.4 | 4.8 ± 1.2 | 22.6 ± 7.4 | 25.1 ± 3.7 | 29.9 ± 13.7 | 84.1 ± 38.3 | 30 / 30 | 12 / 12 |
| 10−6 | 2.5 ± 0.5 | 3.8 ± 0.8 | 25.3 ± 3.6 | 26.3 ± 5.5 | 19.6 ± 9.2 | 42.7 ± 11.4 | 30 / 30 | 12 / 12 |
| 10−7 | 2.1 ± 0.3 | 1.8 ± 0.1 | 37.1 ± 12.7 | 33.8 ± 7.4 | 17.3 ± 11.8 | 15.7 ± 1.5 | 33 / 70 | 2 / 12 |
| 10−8 | 1.9 ± 0.3 | — | 43.8 ± 7.1 | — | 9.9 ± 3.6 | — | 6 / 60 | 0 / 12 |
| 10−9 | 2.3 ± 0.9 | — | 81.0 ± 38.2 | — | 15.0 ± 6.3 | — | 2 / 60 | 0 / 12 |
| 10−10 | — | — | — | — | — | — | 0 / 50 | 0 / 12 |
| SiHa cell line/cells µl−1 | ||||||||
| 2 × 103 | 2.9 ± 0.3 | 4.9 ± 0.6 | 16.9 ± 2.7 | 29.3 ± 1.3 | 42.6 ± 6.2 | 80.1 ± 6.8 | 40 / 40 | 6 / 6 |
| 2 × 102 | 2.8 ± 0.4 | 3.8 ± 1.2 | 18.9 ± 3.4 | 29.3 ± 4.0 | 40.6 ± 14.5 | 52.5 ± 24.8 | 40 / 40 | 6 / 6 |
| 2 × 101 | 2.9 ± 0.3 | 3.7 ± 1.2 | 30.7 ± 9.3 | 33.3 ± 7.9 | 37.5 ± 11.1 | 44.0 ± 16.8 | 39 / 40 | 5 / 6 |
| 2 × 100 | 2.8 ± 0.5 | 3.0 ± 0.4 | 38.0 ± 26.1 | 39.8 ± 1.1 | 35.1 ± 15.5 | 28.0 ± 7.2 | 60 / 70 | 2 / 6 |
| 2 × 10−1 | 2.7 ± 0.5 | — | 70.1 ± 39.1 | — | 39.3 ± 15.0 | — | 4 / 50 | 0 / 6 |
| 2 × 10−2 | — | — | — | — | — | — | 0 / 30 | 0 / 6 |
A comparison of the NASBA results from the HPV 16 sequence and from the SiHa cell line, shows that all parameters display the same trend for microsystems as for conventional methods, except for the ratio between the final and initial fluorescence levels. This ratio is nearly constant for the microchip experiments, but decreases with sample concentration for the conventional experiments. The fluorescense level is determined by the concentration of molecular beacons in the reaction mixture. Theoretically, if the amplification reaches full reactant consumption, the final fluorescense level should be independent of sample concentration, but reached at different times. The overall lower ratio obtained in the microchips could be explained by the enlarged background noise, caused by autofluorescent COC and light scattering from imperfect polymer surfaces. The auto-fluorescence of the microchips was measured to ∼300 mV. Adsorption of reagents to the chamber wall will also contribute to background noise.
The results for the microchips correlate well with the conventional methods (Table 1). When concentrations are reduced, TTP increases, and the average slope decreases, because reagents need more time to find and interact with the targets. Small amounts of target give less amplified material at the beginning of the reaction, and hence the TTP increases. However, very high sample concentrations may slow down the reactions, because of enzymatic inhibition.
The custom-made optical detection system was found to have a detection limit of 10−6 µM for the artificial HPV 16 sequence, and 20 cells µl−1 for the SiHa cell line material. These values are the same as for the conventional Lambda FL600 reader (Table 1). It was possible to detect even lower concentrations in both systems, but the results were inconsistent, most likely due to stochastic sampling effects. The detection limit of the NASBA reaction is dependent on the target of interest, the quality of the RNA samples, and influenced by the design of the primers and the molecular beacon probe. Negative control experiments were run to check for contamination. Because the microchips were only used once, false positive results are only possible if the premixed reaction mixture is contaminated. False negative results could theoretically only arise from contamination with inhibiting agents during microchip fabrication.
The experimental results, based on experiments from 140 microchips × 10 individual reaction channels, including negative controls, are summarized in Table 1. Several factors influence the experimental results and are reflected in the calculated standard deviations. The results show that when the sample concentration of the input target decreases, the standard deviation increases. This could be caused by nonspecific surface binding of the target or pipetting skills. Particularly, nonspecific surface binding is more pronounced for the microchips than for regular microplates, because of a larger surface-to-volume ratio. For the present microchips, this is of great importance, because only three of the four walls forming the reaction chambers were coated with PEG. Experiments with uncoated microchips show complete inhibition of the reaction (data not shown). The PEG coating was in some cases damaged when the membrane was welded to the microchip, at treatment which may change the surface structure and lead to increased nonspecific binding and scattering of the excitation light. Also, BSA acts as a dynamic coating, reducing nonspecific binding of reagents to the channel walls. Volume variations in the pipetting affects the standard deviations for both microchips and microplates. Thus, reagents for individual microchips were mixed separately for each experiment, while for the conventional reactions only two reaction solution were mixed to perform all the experiments. Hence, the exact time for addition of enzymes and insertion of the microchip into the instrument varied between experiments. Also, stochastic sampling variation at lower molecular concentrations may affect the standard deviation.
The microchips used in these experiments had a large dead volume due to the design of the channel network. However, a reduction of dead volume may easily be obtained with a revised design.
Conclusions
Detection of cancer markers using real-time NASBA has been successfully demonstrated. To our knowledge, these are the first results showing detection of mRNA using real-time NASBA within such a microsystem. The detection limits are comparable to those obtained for experiments performed in conventional routine-based laboratory systems, demonstrating that the microchip and its detection system has a potential for diagnostic use in a point-of-care setting.Future microchips could contain more reaction channels, and be combined with multiplexing of several different targets in each of the channels. Simultaneous detection of different targets is possible to identify with multi-parallel reaction channels having integrated different reagents in the channels. The benefits of the present system are reduced reagent consumption, combined with multi-parallel target testing, using only one sample. Hence, less sample material is required, since in many cases the amount of sample material is limited. Finally, an integration of this microchip, with an integrated sample preparation microchip, would constitute a fully automatic, laboratory independant diagnostic system, resulting in an overall time and cost reduction of the whole analysis.
Acknowledgements
This work was partially supported by the Norwegian Research Council. We would like to thank I. Kraus (NorChip AS) for the cultivation of the SiHa cell line from ATCC, and I-R. Johannesen, B. G. Fismen, A. Ferber and H. Schumann-Olsen (SINTEF, Oslo, Norway) for developing the custom-made instrument. Produksjonsteknikk AS (Asker, Norway) designed the robotics, and assembled the instrument.References
- J. M. M. Walboomers, M. V. Jacobs, M. M. Manos, F. X. Bosch, J. A. Kummer, K. V. Shah, P. J. Snijders, J. Peto, C. J. Meijer and N. Muñoz, J. Pathol., 1999, 189, 12–19 CrossRef CAS.
- N. Muñoz, F. X. Bosch, S. de Sanjosé, R. Herrero, X. Castellsagué, K. V. Shah, P. J. F. Snijders and C. J. L. M. Meijer, N. Engl. J. Med., 2003, 348, 518–527 CrossRef.
- D. Jenkins, Curr. Opin. Infect. Dis., 2001, 14, 53–62 Search PubMed.
- J. Compton, Nature, 1991, 350(6313), 91–92 CrossRef CAS.
- G. Leone, H. van Schijndel, B. van Gemen, F. R. Kramer and C. D. Schoen, Nucleic Acids Res., 1998, 26(9), 2150–2155 CrossRef CAS.
- I. Kraus, T. Molden, L. E. Ernø, H. Skomedal, F. Karlsen and B. Hagmar, Br. J. Cancer, 2004, 90(7), 1407–1413 CrossRef CAS.
- K. S. Cuschieri, M. J. Whitley and H. A. Cubie, J. Med. Virol., 2004, 73, 65–70 CrossRef CAS.
- H. G. Elias, An introduction to polymer science, 1997, VCH, NY Search PubMed.
- K. S. Drese, O. Soerensen, L. Solli and A. Gulliksen, smallTalk2003, The Microfluidics, Microarrays and BioMEMS conference, July 13–16, 2003, San Jose, CA, USA, p. 69, Association for Laboratory Automation, http://www.labautomation.org Search PubMed.
- A. Gulliksen, L. Solli, F. Karlsen, H. Rogne, E. Hovig, T. Nordstrøm and R. Sirevåg, Anal. Chem., 2004, 76, 9–14 CrossRef CAS.
- S. Syrjanen, P. Partanen, R. Mantyjarvi and K. Syrjanen, J. Virol. Methods, 1988, 19, 225–238 CrossRef CAS.
- A. Mincheva, L. Gissmann and H. zur Hausen, Med. Microbiol. Immunol., 1987, 176(5), 245–256 CrossRef CAS.
- H. B. Heiles, E. Genersch, C. Kessler, R. Neumann and H. J. Eggers, Biotechniques, 1988, 6(10), 978–981 CAS.
- R. Boom, J. A. Sol, M. M. M. Salimans, C. L. Jansen, P. M. E. Wertheimvandillen and J. Vandernoordaa, J Clin. Microbiol., 1990, 28(3), 495–503 CAS.
- H. G. M. Niesters, Methods, 2001, 25, 419–429 Search PubMed.
- M. P. de Baar, M. W. van Dooren, E. de Rooij, M. Bakker, B. van Gemen, J. Goudsmit and A. de Ronde, J. Clin. Microbiol., 2001, 39(4), 1378–1384 CrossRef CAS.
- I. Kraus, NorChip AS, Norway, personal communication.
| This journal is © The Royal Society of Chemistry 2005 |
