Structurally divergent enantioselective synthesis of benzofuran fused azocine derivatives and spiro-cyclopentanone benzofurans enabled by sequential catalysis†
Abstract
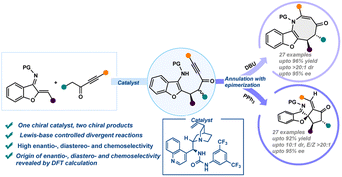
An important objective in organic synthesis and medicinal chemistry is the capacity to access structurally varied and complex molecules rapidly and affordably from easily available starting materials. Herein, a protocol for the structurally divergent synthesis of benzofuran fused azocine derivatives and spiro-cyclopentanone benzofurans has been developed via chiral bifunctional urea catalyzed reaction between aurone-derived α,β-unsaturated imine and ynone followed by switchable divergent annulation reactions by Lewis base catalysts (DBU and PPh3) with concomitant epimerization. The skeletally diversified products were formed in high yields with high diastereo- and enantioselectivities. Computational analysis with DFT and accurate DLPNO-CCSD(T) has been employed to gain deeper insights into mechanistic intricacies and investigate the role of chiral and Lewis base catalysts in skeletal diversity.
- This article is part of the themed collection: Most popular 2023 organic chemistry articles


 Please wait while we load your content...
Please wait while we load your content...