A high-throughput effector screen identifies a novel small molecule scaffold for inhibition of ten-eleven translocation dioxygenase 2†
Abstract
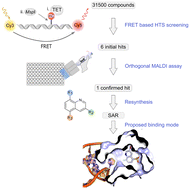
Ten-eleven translocation dioxygenases (TETs) are the erasers of 5-methylcytosine (mC), the central epigenetic regulator of mammalian DNA. TETs convert mC to three oxidized derivatives with unique physicochemical properties and inherent regulatory potential, and it initializes active demethylation by the base excision repair pathway. Potent small molecule inhibitors would be useful tools to study TET functions by conditional control. To facilitate the discovery of such tools, we here report a high-throughput screening pipeline and its application to screen and validate 31.5k compounds for inhibition of TET2. Using a homogenous fluorescence assay, we discover a novel quinoline-based scaffold that we further validate with an orthogonal semi-high throughput MALDI-MS assay for direct monitoring of substrate turnover. Structure–activity relationship (SAR) studies involving >20 derivatives of this scaffold led to the identification of optimized inhibitors, and together with computational studies suggested a plausible model for its mode of action.


 Please wait while we load your content...
Please wait while we load your content...