Boosting the activity of Mizoroki–Heck cross-coupling reactions with a supramolecular palladium catalyst favouring remote Zn⋯pyridine interactions†
Abstract
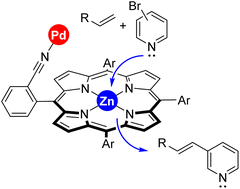
Transition metal catalysis benefitting from supramolecular interactions in the secondary coordination sphere in order to pre-organize substrates around the active site and reach a specific selectivity typically occurs under long reaction times and mild reaction temperatures with the aim to maximize such subtle effects. Herein, we demonstrate that the kinetically labile Zn⋯N interaction between a pyridine substrate and a zinc–porphyrin site serving for substrate binding is a unique type of weak interaction that enables identification of supramolecular effects in transition metal catalysis after one hour at a high reaction temperature of 130 °C. Under carefully selected reaction conditions, supramolecularly-regulated palladium-catalyzed Mizoroki–Heck reactions between 3-bromopyridine and terminal olefins (acrylates or styrenes) proceeded in a more efficient manner compared to the non-supramolecular version. The supramolecular catalysis developed here also displayed interesting substrate-selectivity patterns.
- This article is part of the themed collection: Harnessing non-covalent interactions for synthesis and catalysis


 Please wait while we load your content...
Please wait while we load your content...