Atmospheric chemistry of (Z)- and (E)-1,2-dichloroethene: kinetics and mechanisms of the reactions with Cl atoms, OH radicals, and O3
Abstract
Smog chambers interfaced with in situ FT-IR detection were used to investigate the kinetics and mechanisms of the Cl atom, OH radical, and O3 initiated oxidation of (Z)- and (E)-1,2-dichloroethene (CHCl![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCl) under atmospheric conditions. Relative and absolute rate methods were used to measure k(Cl + (Z)-CHCl
CHCl) under atmospheric conditions. Relative and absolute rate methods were used to measure k(Cl + (Z)-CHCl![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCl) = (8.80 ± 1.75) × 10−11, k(Cl + (E)-CHCl
CHCl) = (8.80 ± 1.75) × 10−11, k(Cl + (E)-CHCl![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCl) = (8.51 ± 1.69) × 10−11, k(OH + (Z)-CHCl
CHCl) = (8.51 ± 1.69) × 10−11, k(OH + (Z)-CHCl![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCl) = (2.02 ± 0.43) × 10−12, k(OH + (E)-CHCl
CHCl) = (2.02 ± 0.43) × 10−12, k(OH + (E)-CHCl![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCl) = (1.94 ± 0.43) × 10−12, k(O3 + (Z)-CHCl
CHCl) = (1.94 ± 0.43) × 10−12, k(O3 + (Z)-CHCl![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCl) = (4.50 ± 0.45) × 10−21, and k(O3 + (E)-CHCl
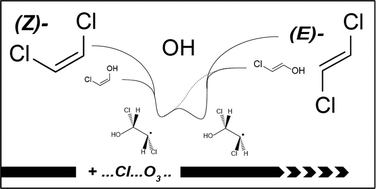
CHCl) = (4.50 ± 0.45) × 10−21, and k(O3 + (E)-CHCl![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCl) = (1.02 ± 0.10) × 10−19 cm3 molecule−1 s−1 in 700 Torr of N2/air diluent at 298 ± 2 K. Pressure dependencies for the Cl atom reaction kinetics were observed for both isomers, consistent with isomerization occurring via Cl atom elimination from the chemically activated CHCl–CHCl–Cl adduct. The observed products from Cl initiated oxidation were HC(O)Cl (117–133%), CHCl2CHO (29–30%), and the corresponding CHCl
CHCl) = (1.02 ± 0.10) × 10−19 cm3 molecule−1 s−1 in 700 Torr of N2/air diluent at 298 ± 2 K. Pressure dependencies for the Cl atom reaction kinetics were observed for both isomers, consistent with isomerization occurring via Cl atom elimination from the chemically activated CHCl–CHCl–Cl adduct. The observed products from Cl initiated oxidation were HC(O)Cl (117–133%), CHCl2CHO (29–30%), and the corresponding CHCl![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCl isomer (11–20%). OH radical initiated oxidation gives HC(O)Cl as a major product. For reaction of OH with (E)-CHCl
CHCl isomer (11–20%). OH radical initiated oxidation gives HC(O)Cl as a major product. For reaction of OH with (E)-CHCl![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCl, (Z)-CHCl
CHCl, (Z)-CHCl![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCl was also observed as a product. A significant chlorine atom elimination channel was observed experimentally (HCl yield) and supported by computational results. Photochemical ozone creation potentials of 12 and 11 were estimated for (Z)- and (E)-CHCl
CHCl was also observed as a product. A significant chlorine atom elimination channel was observed experimentally (HCl yield) and supported by computational results. Photochemical ozone creation potentials of 12 and 11 were estimated for (Z)- and (E)-CHCl![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCl, respectively. Finally, an empirical kinetic relationship is explored for the addition of OH radicals or Cl atoms to small alkenes. The results are discussed in the context of the atmospheric chemistry of (Z)- and (E)-CHCl
CHCl, respectively. Finally, an empirical kinetic relationship is explored for the addition of OH radicals or Cl atoms to small alkenes. The results are discussed in the context of the atmospheric chemistry of (Z)- and (E)-CHCl![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCl.
CHCl.


 Please wait while we load your content...
Please wait while we load your content...