An engineered biosynthetic–synthetic platform for production of halogenated indolmycin antibiotics†
Abstract
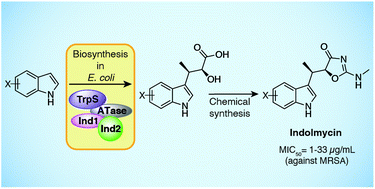
Indolmycin is an antibiotic from Streptomyces griseus ATCC 12648 with activity against Helicobacter pylori, Plasmodium falciparum, and methicillin-resistant Staphylococcus aureus. Here we describe the use of the indolmycin biosynthetic genes in E. coli to make indolmycenic acid, a chiral intermediate in indolmycin biosynthesis, which can then be converted to indolmycin through a three-step synthesis. To expand indolmycin structural diversity, we introduce a promiscuous tryptophanyl-tRNA synthetase gene (trpS) into our E. coli production system and feed halogenated indoles to generate the corresponding indolmycenic acids, ultimately allowing us to access indolmycin derivatives through synthesis. Bioactivity testing against methicillin-resistant Staphylococcus aureus showed modest antibiotic activity for 5-, 6-, and 7-fluoro-indolmycin.


 Please wait while we load your content...
Please wait while we load your content...