Metal-free oxidative coupling of arylmethylamines with indoles: a simple, environmentally benign approach for the synthesis of 3,3′-bis(indolyl)methanes†
Abstract
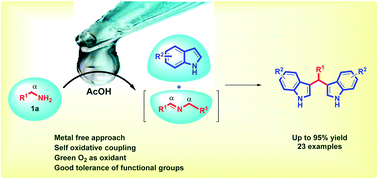
The efficient metal-free oxidative coupling of arylmethylamines with indoles has been developed using molecular oxygen as a green oxidant. The present reaction provides a novel route towards the synthesis of 3,3′-bis(indolyl)methanes in excellent yields of up to 95% via C–C and C–N bond formation. This attractive and environmentally friendly one-pot protocol is a simple procedure that features inexpensive acetic acid as the catalyst and molecular oxygen as the sole oxidant, and it supports a wide substrate scope with the good tolerance of functional groups.


 Please wait while we load your content...
Please wait while we load your content...