Design rules for encapsulating proteins into complex coacervates†
Abstract
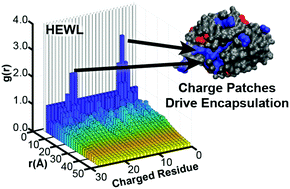
We investigated the encapsulation of the model proteins bovine serum albumin (BSA), human hemoglobin (Hb), and hen egg white lysozyme (HEWL) into two-polymer complex coacervates as a function of polymer and solution conditions. Electrostatic parameters such as pH, protein net charge, salt concentration, and polymer charge density can be used to modulate protein uptake. While the use of a two-polymer coacervation system enables the encapsulation of weakly charged proteins that would otherwise require chemical modification to facilitate electrostatic complexation, we observed significantly higher uptake for proteins whose structure includes a cluster of like-charged residues on the protein surface. In addition to enhancing uptake, the presence of a charge patch also increased the sensitivity of the system to modulation by other parameters, including the length of the complexing polymers. Lastly, our results suggest that the distribution of charge on a protein surface may lead to different scaling behaviour for both the encapsulation efficiency and partition coefficient as a function of the absolute difference between the protein isoelectric point and the solution pH. These results provide insight into possible biophysical mechanisms whereby cells can control the uptake of proteins into coacervate-like granules, and suggest future utility in applications ranging from medicine and sensing to remediation and biocatalysis.
- This article is part of the themed collection: Soft Matter Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...