pH sensitivity of interfacial electron transfer at a supported graphene monolayer†
Abstract
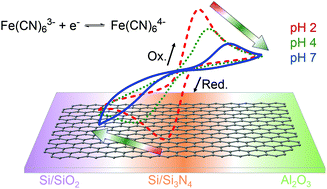
Electrochemical devices based on a single graphene monolayer are often realized on a solid support such as silicon oxide, glassy carbon or a metal film. Here, we show that, with graphene on insulating substrates, the kinetics of the electron transfer at graphene with various redox active molecules is dictated by solution pH for electrode reactions that are not proton dependent. We attribute the origin of this unusual phenomenon mainly to electrostatic effects between dissolved/dissociated redox species and the interfacial charge due to trace amounts of ionizable groups at the supported graphene–liquid interface. Cationic redox species show higher electron transfer rates at basic pH, while anionic species undergo faster electron transfer at acidic pH. Although this behavior is observed on graphene on three different insulating substrates, the strength of this effect appears to differ depending on the surface charge density of the underlying substrate. This finding has important implications for the design of electrochemical sensors and electrocatalysts based on graphene monolayers.


 Please wait while we load your content...
Please wait while we load your content...