An experimental and computational study of the effect of aqueous solution on the multiphoton ionisation photoelectron spectrum of phenol†
Abstract
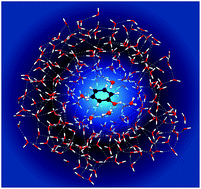
We revisit the photoelectron spectroscopy of aqueous phenol in an effort to improve our understanding of the impact of inhomogeneous broadening and inelastic scattering on solution-phase photoelectron spectra. Following resonance-enhanced multiphoton ionisation via the 11ππ* and 11πσ* states of phenol, we observe 11ππ*–D0/D1 ionisation and competing direct S0–D0/D1 ionisation. Following resonance-enhanced multiphoton ionisation via the 21ππ* state, we observe the signature of solvated electrons. By comparing the photoelectron spectra of aqueous phenol with those of gas-phase phenol, we find that inelastic scattering results in peak shifts with similar values to those that have been observed in photoelectron spectra of solvated electrons, highlighting the need for a robust way of deconvoluting the effect of inelastic scattering from liquid-phase photoelectron spectra. We also present a computational strategy for calculating vertical ionisation energies using a quantum-mechanics/effective fragmentation potential (QM/EFP) approach, in which we find that optimising the configurations obtained from molecular dynamics simulations and using the [phenol·(H2O)5]QM[(H2O)n≥250]EFP (B3LYP/aug-cc-pvdz) method gives good agreement with experiment.
- This article is part of the themed collection: Quantum effects in complex systems


 Please wait while we load your content...
Please wait while we load your content...