Metal–ligand cooperative C–O bond cleavage of propargylic alcohol with protic pyrazole complexes of ruthenium†
Abstract
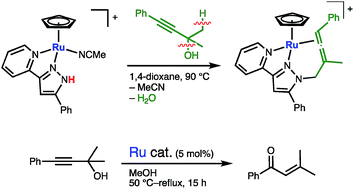
The C–O bond cleavage of a propargylic alcohol, 1,1-dimethyl-3-phenylprop-2-yn-1-ol (3), by metal–ligand cooperation was investigated. The reactions of a naphthalene complex [CpRu(η6-C10H8)]PF6 (Cp = η5-C5H5) with 5-R-3-(pyrid-2-yl)pyrazoles (R-LH; R = Ph, CF3) in acetonitrile afforded the starting metal–ligand bifunctional ruthenium complexes [CpRu(R-LH)(MeCN)]PF6 (1a, R = Ph; 1b, R = CF3) featuring an N–N chelate protic pyrazole. The treatment of the CF3-substituted pyrazole complex 1b with 3 in 1,2-dichloroethane at 50 °C resulted in the formation of the η3-butadienyl complex 5. Meanwhile, the corresponding reaction of the phenylpyrazole complex 1a in 1,4-dioxane at 90 °C gave the N-allenylmethylpyrazole complex 6. The C–O and C–H bond cleavage of 3 in these reactions can be ascribed to the metal–ligand cooperation: initial formation of an η3-propargyl complex assisted by NH⋯O hydrogen bonding and following C–H deprotonation by the neighboring pyrazolato ligand. On the other hand, in boiling methanol, the protic pyrazole complex 1a catalyzed the C–O bond cleavage of 3, which resulted in catalytic isomerization of 3 to an α,β-unsaturated enone, 3-methyl-1-phenylbut-2-en-1-one (8). The control experiments using non-protic and isocyanide ruthenium complexes indicated that both a labile nitrile ligand on the metal and the protic cooperating ligand are crucial for the catalysis.
- This article is part of the themed collection: Mechanistic processes in organometallic chemistry


 Please wait while we load your content...
Please wait while we load your content...