Barrier kinetics of adsorption–desorption of alcohol monolayers on water under constant surface tension†
Abstract
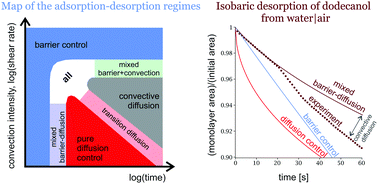
The desorption of spread decanol and dodecanol monolayers at controlled constant surface tension is shown to proceed under mixed barrier-diffusion control; the role of the convective diffusion is also discussed. The desorption rate is measured as a function of the density of the monolayer and the temperature. The rate of barrier desorption increases as the monolayer approaches the collapse point, reaching an infinite value. The average desorption time of an adsorbed dodecanol molecule increases linearly with the area per molecule, and is phase-specific – it is higher for the liquid condensed state of the monolayer than for the liquid expanded. The desorption rate increases with temperature; the activation energy for desorption is independent of the compression and the surface phase. The increase of the intensity of convection is shown to produce a vanishingly thin diffusion layer and causes the desorption to proceed under pure barrier control. A schematic map of the adsorption–desorption regimes acting as a function of time and intensity of the convection is constructed. General expressions for the rate of adsorption and desorption of alcohols are formulated.


 Please wait while we load your content...
Please wait while we load your content...