An unprecedented tandem synthesis of fluorescent coumarin-fused pyrimidines via copper-catalyzed cross-dehydrogenative C(sp3)–N bond coupling†
Abstract
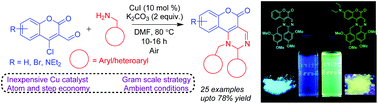
An efficient, one-pot Cu-catalyzed tandem synthesis of fluorescent 1-benzyl-2-phenyl-1,2-dihydro-5H-chromeno[4,3-d]pyrimidin-5-ones from 4-chloro-3-formylcoumarin and benzylamines was developed by in situ intramolecular cross-dehydrogenative C(sp3)–N bond formation in moderate-to-good yields under ligand-free ambient conditions. This synthesis was easily scalable, and the generality of the substrates was established. These coumarin-fused pyrimidines exhibited interesting photo-physical properties and high quantum yields, and would be potential candidates for facilitating suitable studies in medicinal chemistry and materials science.


 Please wait while we load your content...
Please wait while we load your content...