Single-file transport of water through membrane channels
Abstract
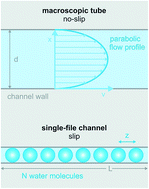
Water at interfaces governs many processes on the molecular scale from electrochemical and enzymatic reactions to protein folding. Here we focus on water transport through proteinaceous pores that are so narrow that the water molecules cannot overtake each other in the pore. After a short introduction into the single-file transport theory, we analyze experiments in which the unitary water permeability, pf, of water channel proteins (aquaporins, AQPs), potassium channels (KcsA), and antibiotics (gramicidin-A derivatives) has been obtained. A short outline of the underlying methods (scanning electrochemical microscopy, fluorescence correlation spectroscopy, measurements of vesicle light scattering) is also provided. We conclude that pf increases exponentially with a decreasing number NH of hydrogen bond donating or accepting residues in the channel wall. The variance in NH is responsible for a more than hundredfold change in pf. The dehydration penalty at the channel mouth has a smaller effect on pf. The intricate link between pf and the Gibbs activation energy barrier, ΔG‡t, for water flow suggests that conformational transitions of water channels act as a third determinant of pf.
- This article is part of the themed collection: Artificial Water Channels


 Please wait while we load your content...
Please wait while we load your content...