Hyperpolarised NMR to follow water proton transport through membrane channels via exchange with biomolecules
Abstract
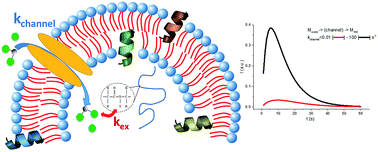
Water uptake in vesicles and the subsequent exchange between water protons and amide –NH protons in amino acids can be followed by a new, highly sensitive, type of magnetic resonance spectroscopy: dynamic nuclear polarisation (DNP)-enhanced NMR in the liquid state. Water hydrogen atoms are detected prior to and after their transfer to molecular sites in peptides and proteins featuring highly-accessible proton-exchangeable groups, as is the case for the –NH groups of intrinsically disordered proteins. The detected rates for amide proton–water proton exchange can be modulated by membrane-crossing rates, when a membrane channel is interposed. We hyperpolarised water proton spins via dynamic nuclear polarisation followed by sample dissolution (d-DNP) and transferred the created polarisation to –NH groups with high solvent accessibility in an intrinsically disordered protein domain. This domain is the membrane anchor of c-Src kinase, whose activity controls cell proliferation. The hindrance of effective water proton transfer rate constants observed in free solvent when a membrane-crossing step is involved is discussed. This study aims to assess the feasibility of recently-introduced hyperpolarised (DNP-enhanced) NMR to assess water membrane crossing dynamics.
- This article is part of the themed collection: Artificial Water Channels


 Please wait while we load your content...
Please wait while we load your content...