Nanoengineering ABO3 active sites from low-energy routes (TX100-stabilised water-in-oil microemulsions, surface segregation and surface complexation on colloidal AlOOH/sol–gel Al2O3 surfaces) for pollution control catalysis
Abstract
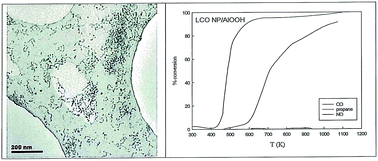
It is shown that water-in-oil microemulsions (m/e or μE) can produce BaCeO3 (BCO) and LaCoO3 (LCO) precursors. The nanoparticles (NPs) adsorb on AlOOH sols, in much the same way as Turkevich previously immobilised platinum group metal sols. BCO is active in CO and propane oxidation and NO removal under stoichiometric exhaust conditions, but LCO is a better oxidation catalyst. Activity was also seen when Ba,Ce and La,Co are inserted into/segregate at the surface of AlOOH/Al2O3. However, there is only formation of low levels of BCO, CAIO3 (CAO), LCO and LaAIO3 (LAO) perovskites, along with aluminates and separate oxides. The complexing of cations by AlOOH surface-held oxalate ions, albeit with different efficiencies, has also been explored. All three routes yield active catalysts with micro-domains of crystallinity; microemulsions produce the best defined perovskite NPs, but even those from surface segregation have higher turnover numbers than traditional Pt catalysts. Perovskite NPs may open up green chemistry for air pollution control that is consistent with a circular economy.
- This article is part of the themed collection: Designing Nanoparticle Systems for Catalysis

 Please wait while we load your content...
Please wait while we load your content...