Rapid access to diverse, trifluoromethyl-substituted alkenes using complementary strategies†
Abstract
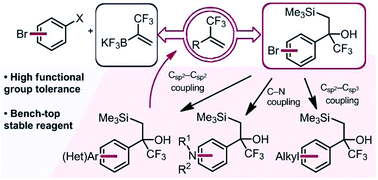
Two synergistic approaches to the facile assembly of complex α-trifluoromethyl alkenes are described. Using α-trifluoromethyl-β-silyl alcohols as masked trifluoromethyl alkenes, cross-coupling or related functionalization processes at distal electrophilic sites can be executed without inducing Peterson elimination. Subsequent Lewis acidic activation affords functionalized α-trifluoromethyl alkenes. Likewise, the development of a novel α-trifluoromethylvinyl trifluoroborate reagent complements this approach and allows a one-step cross-coupling of (hetero)aryl halides to access a broad array of complex α-trifluoromethyl alkenes.


 Please wait while we load your content...
Please wait while we load your content...