Synthesis, and single crystal structure of fully-substituted polynitrobenzene derivatives for high-energy materials†
Abstract
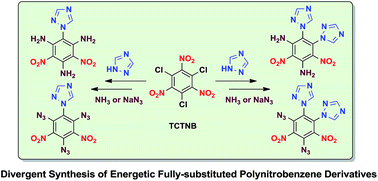
New energetic fully-substituted polynitrobenzene derivatives were synthesized via the reaction of 1,3,5-trichloro-2,4,6-trinitrobenzene (TCTNB) with 1,2,4-triazole followed by the nucleophilic substitution of the halo groups by aqueous ammonia or sodium azide. These compounds were charactered by 1H NMR, 13C NMR and HRMS. Additionally, the structures of amino derivatives 6, 8 and azido derivative 7 were further confirmed by single crystal X-ray diffraction analysis. Their decomposition temperatures and impact sensitivities were also determined. The derivative 1,2-di-1H-triazol-4,6-diamino-3,5-dinitrobenzene (8) exhibits good thermal stability (Td = 314 °C) and low impact sensitivity (IS = 30 J), which are both superior to that of TNT (Td = 295 °C, IS = 15 J). These synthesized compounds showed high heat of formation ranging from 31.77 to 1014.68 kJ mol−1, and reasonable detonation velocities and pressures.


 Please wait while we load your content...
Please wait while we load your content...