Phosphine-catalyzed Friedel–Crafts reaction of naphthols with para-quinone methides: expedient access to triarylmethanes†
Abstract
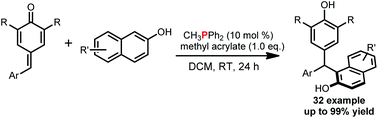
We developed a novel phosphine-catalyzed Friedel–Crafts reaction of naphthols with para-quinone methides (p-QMs). This reaction provided a promising method for the synthesis of triarylmethanes, which widely exist in natural products and in molecules which have been shown to have biological and pharmacological activity.


 Please wait while we load your content...
Please wait while we load your content...