Bismuth oxyiodide coupled with bismuth nanodots for enhanced photocatalytic bisphenol A degradation: synergistic effects and mechanistic insight†
Abstract
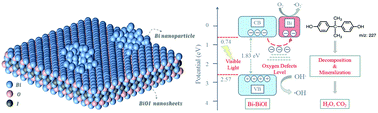
Bismuth based semiconductor photocatalysts are being generated as promising materials for photocatalysis. In this work, hydrothermal methods have been utilized to synthesize a bismuth oxyiodide semiconductor with deposited Bi nanodots (Bi-BiOI), which could create oxygen defects and accelerate photoinduced charge migration simultaneously. The resulting Bi-BiOI strongly demonstrates the high photocatalytic performance for bisphenol A and methylene blue degradation under visible light. 86% of BPA was degraded after an irradiation time of 4 hours. Electrospray ionization mass spectrometry was employed to detect the evolution of intermediates formed during the decomposition process of bisphenol A, and the following results suggested complete bisphenol A mineralization. Additionally, electron paramagnetic resonance results revealed the production of free radicals and the presence of oxygen vacancies. Furthermore, a distinctively increased photocurrent response and photoluminescence decay dynamics demonstrate that the interface between the Bi nanodots and BiOI semiconductor promotes the separation and migration of photoinduced electron–hole pairs. The lower valence band value (2.57 eV) of Bi-BiOI presented a higher oxidation potential, thus the production of hydroxyl radicals could be promoted considerably. Based on the experimental results, factors such as oxygen vacancies, effective charge migration, suppressed photoinduced electron–hole pair recombination and a high Bi-BiOI oxidation potential would result in advanced free radical production capacity, thereby enhancing the photocatalytic efficiency. The findings of our work will contribute to the fabrication of metal nanodot deposited semiconductor photocatalysts and pave the way for the utilization of advanced oxidation technology.


 Please wait while we load your content...
Please wait while we load your content...