Decarboxylative Csp3–Csp3 coupling for benzylation of unstable ketone enolates: synthesis of p-(acylethyl)phenols†
Abstract
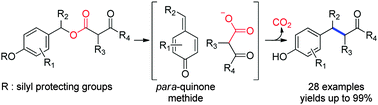
A new decarboxylative Csp3–Csp3 coupling approach for the benzylation of ketone enolates has been developed. A variety of raspberry ketone derivatives were conveniently synthesized in good to excellent yields under mild conditions. A crossover reaction shed light on the mechanism of this tandem reaction.

 Please wait while we load your content...
Please wait while we load your content...