Retracted Article: Spectrin-like domain 2 of DRP2 serves as a novel binding region for the NLS2 and 3 sub-domains of L-periaxin
Abstract
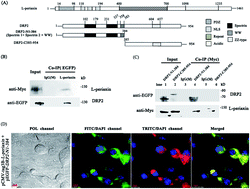
L-periaxin is an important scaffolding protein that is expressed in Schwann cells and lens fiber cells. In Schwann cells, loss or mutation of the PRX gene disrupts compact peripheral myelin and causes severe demyelination neuropathy. DRP2 is the only reported protein to interact with periaxin to form the L-periaxin–DRP2–dystroglycan (PDG) complex, which can provide structural and signaling functions by linking the extracellular matrix to the Schwann cell cytoskeleton. In this report, the interaction between L-periaxin and DRP2 was further investigated by bimolecular fluorescence complementation (BiFC), GST pull-down, CO-IP, and fluorescence spectroscopy. Results demonstrated that spectrin-like domain 2 of DRP2 played a critical role in the complex of DRP2 and L-periaxin. Furthermore, the DRP2 spectrin-like domain 2 only interacted with the NLS2 and NLS3 sub-domains in L-periaxin. These data revealed a previously unknown binding model between DRP2 and L-periaxin.

 Please wait while we load your content...
Please wait while we load your content...