The energetic viability of an unexpected skeletal rearrangement in cyclooctatin biosynthesis†
Abstract
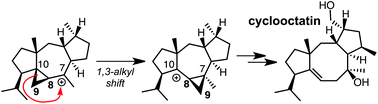
Results of density functional theory calculations on possible mechanisms for formation of the diterpenoid cyclooctatin are described. These results are consistent with the involvement of an unexpected 1,3-alkyl shift that interconverts two cyclopropylcarbinyl carbocations and interchanges the positions of two carbon atoms in an 8-membered ring. Predictions for future experiments to provide further support of this mechanism also are described.

 Please wait while we load your content...
Please wait while we load your content...