Retracted Article: Synthesis of octahedral, truncated octahedral, and cubic Rh2Ni nanocrystals and their structure–activity relationship for the decomposition of hydrazine in aqueous solution to hydrogen†
Abstract
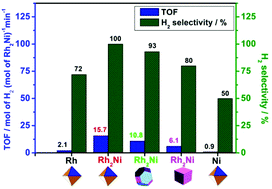
We developed a co-reduction method to synthesize octahedral, truncated octahedral, and cubic Rh2Ni nanocrystals. The shape/size distribution, structural characteristics, and composition of the Rh2Ni nanocrystals are investigated, and their possible formation mechanism at high temperatures in margaric acid/1-aminoheptadecane solution in the presence of tetraethylgermanium and borane trimethylamine complexes is proposed. A preliminary probing of the structure–activity dependence of the surface “clean” Rh2Ni nanocrystals supported on carbon towards hydrazine (N2H4) in aqueous solution dehydrogenation revealed that the higher the percentage of {111} facets, the higher is the activity and H2 selectivity of the nanocrystals. This result was attributed to the {111} facets not only introducing more basic sites, but also weakening the interaction between the produced adspecies (including H2 and NHx) and surface metal atoms in comparison with those of {100} facets. Furthermore, the as-prepared Rh2Ni nanooctahedra exhibited 100% H2 selectivity and high activity at room temperature for H2 generation via N2H4 decomposition. The activation energy of the Rh2Ni nanooctahedra was 41.6 ± 1.2 kJ mol−1. The Rh2Ni nanooctahedra were stable catalysts for the hydrolytic dehydrogenation of N2H4, providing 27 723 total turnovers in 30 h. Our work provides a new perspective concerning the possibility of constructing hydrogen-producing systems based on N2H4 and surface “clean” Rh2Ni nanocrystal catalysts with defined shapes supported on carbon that possess a competitive performance in comparison with NaBH4 and NH3BH3 hydrogen-producing systems for fuel cell applications.

 Please wait while we load your content...
Please wait while we load your content...