The transformation from amorphous iron phosphate to sodium iron phosphate in sodium-ion batteries
Abstract
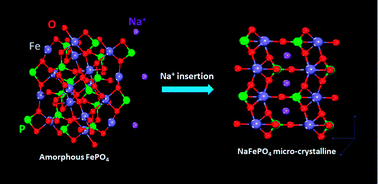
In this article, the structure and electrochemical performance of sodiated iron phosphate (FePO4) synthesized by the micro-emulsion technique have been investigated by X-ray diffraction (XRD), high resolution transmission electron microscopy (HRTEM) and electrochemical measurement. The results reveal that amorphous FePO4 could be transformed into crystallite sodium iron phosphate (NaFePO4) during electrochemical sodiation. Furthermore, the results of electrochemical testing show that the initial specific-discharge capacity of FePO4 is 142 mA h g−1, and it still delivers a reversible capacity of 130.8 mA h g−1 after 120 cycles. The discharge capacities could attain values of 142 mA h g−1, 119.1 mA h g−1, 91.5 mA h g−1 and 63.5 mA h g−1 at 0.1 C, 0.2 C, 0.5 C and 1 C, respectively. These findings have indicated that NaFePO4 has been formed during the electrochemical process and that amorphous structured FePO4 is one of the most promising “host” materials.

 Please wait while we load your content...
Please wait while we load your content...