Syntheses, structures, optical properties and biological activities of bimetallic complexes†
Abstract
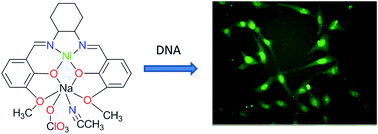
A bimetallic Schiff base-metal complex [Ni(L)Na(CH3CN)(ClO4)] (1) and a coordination polymer [Zn2(L)(N3)2]n (2) (H2L = N,N′-bis(3-methoxysalicylidehydene)cyclohexane-1,2-diamine) have been synthesized and characterised by IR, elemental analyses and single crystal X-ray diffraction. The solid state structure reveals that complex 1 is a heterometallic Ni(II)–Na(I) compound and complex 2 is a homometallic coordination polymer of Zn(II) bridged by azide anions. The ligand coordinates to the Ni(II) ion or one of the Zn(II) ions through two imine nitrogen atoms and two phenolic oxygen atoms in 1 and 2, respectively. The Na(I) ion is coordinated by four oxygen atoms of the phenoxo and methoxy groups of the ligand. This is further bonded to perchlorate in 1. The optical properties of 1 and 2 have been investigated. The cellular toxicity, cellular uptake and DNA binding of complex 1 have been explored.

 Please wait while we load your content...
Please wait while we load your content...