Anion exchange membranes by bromination of benzylmethyl-containing poly(fluorene ether sulfone)s
Abstract
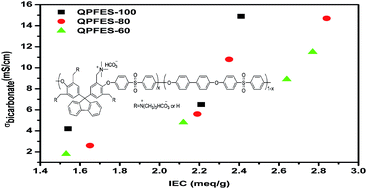
Fluorene-containing anion exchange membranes were synthesized via the bromination reaction of poly(sulfone)s derived from 9,9-bis(3,5-dimethyl-4-hydroxyphenyl)fluorene (DMHPF), 4-fluorophenyl sulfone and 4,4′-biphenol, quaternization reaction using trimethylamine, and ion exchange; then the properties of these AEMs were fully characterized, and the structure–property relationship regarding this series of AEMs was elucidated. Brominated poly(fluorene ether sulfone)s (BrPFES) were characterized using proton nuclear magnetic resonance (1H NMR), and the bromination conversion, degree of functionalization (DF), and conversion of benzylmethyl groups were calculated. BrPFES was then quaternized by heterogeneous amination using trimethylamine, and then converted to quaternary ammonium bicarbonate by ion exchange. The properties of these AEMs were studied in terms of water uptakes, conductivities, swelling ratios, and mechanical properties. Compared to their homopolymer counterparts with similar ion exchange capacities (IEC), AEMs based on copolymers showed slightly lower conductivities but much lower water uptakes and swelling ratios, which can be explained by the fact that the continuation of hydrophilic domains in copolymers was interrupted by the incorporation of hydrophobic 4,4′-biphenol segments.

 Please wait while we load your content...
Please wait while we load your content...