The reduction effect of dietary flavone C- and O-glycosides on the formation of acrylamide and its correlation and prediction with the antioxidant activity of Maillard reaction products
Abstract
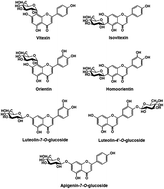
The effect of dietary flavone C- and O-glycosides on the formation of acrylamide contaminants has been investigated in the present work. Flavone glycosides were added in different concentrations, and the acrylamide levels were quantified in a potato-based equimolar asparagine–reducing sugar model system via microwave heating. Results indicated a non-linear relationship between the addition levels of flavone glycosides and inhibitory rates of acrylamide formation in the Maillard reaction. The maximum inhibitory rate (25.3–63.5%) was observed when the addition levels of all flavone glycosides was 10−9 mol L−1. A structure–activity analysis of the ability of flavonoids to reduce the formation of acrylamide revealed that both the number and position of phenol hydroxyl functional groups play an important role in the inhibition ability of flavonoids. Furthermore, flavone C-glycosides are more effective at inhibiting the formation of acrylamide than flavone O-glycosides, although they share the same aglycone structure. The rate of inhibition of acrylamide formation correlated well with changes in trolox equivalent antioxidant capacity (ΔTEAC) measured by DPPH (R2 = 0.934), ABTS (R2 = 0.897) or FRAP (R2 = 0.912) assay. Using ΔTEAC as variables, a multiple linear regression (MLR) model could effectively serve as a predictive tool for estimating the reduction of acrylamide by flavone glycosides during microwave heat processing. These observations are important for our understanding and future development of agents that might decrease the formation of this hazardous toxin.

 Please wait while we load your content...
Please wait while we load your content...