Chemistry of aromatic polythioesters and polydithioesters†
Abstract
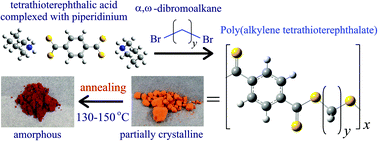
Aromatic polythioesters (abbreviated as PyTS2) and polydithioesters (PyTS4) (y: the number of methylene units = 2–5), analogues of the common aromatic polyesters such as poly(ethylene terephthalate) and poly(butylene terephthalate), have been synthesized and characterized in terms of solubility, crystallinity, glass transition, melting, thermal decomposition, molecular motion, and thermal transition. Conformational characteristics of the S–(CH2)y–S bond sequences of the two groups of polymers were investigated through NMR and single crystal X-ray diffraction experiments as well as molecular orbital calculations for their model compounds. The synthetic scheme developed previously for P2TS4 has been successfully extended to its higher homologues, PyTS4 (y = 3–5). The structure of the monomer, tetrathioterephthalic acid (S4TPA) complexed with piperidinium (Pip), was determined, and the molar ratio of S4TPA : Pip was evaluated as 1 : 2 from elemental analysis and density functional theoretical simulations for its 1H and 13C NMR spectra. Two polydithioesters, P3TS4 and P4TS4, show an endothermic change around 130–150 °C. By X-ray diffraction and solid state NMR experiments, the endotherm was proved to be due to an irreversible crystalline-to-amorphous transition.

 Please wait while we load your content...
Please wait while we load your content...