Synthesis of l-rhamnose derived chiral bicyclic triazoles as novel sodium-glucose transporter (SGLT) inhibitors†
Abstract
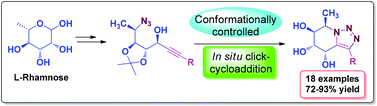
Herein we describe the synthesis of a series of novel fused bicyclic 1,2,3-triazoles from commercially available, natural deoxy sugar, L-rhamnose. The key reactions involved are (i) Zn(OTf)2 catalyzed enantioselective alkynylation of L-rhamnose derived azidoaldehyde and (ii) deprotection of the acid sensitive 1,2-isopropylidene group followed by in situ intramolecular click-cycloaddition of azidoalkynols. Some compounds exhibit excellent sodium-glucose transporter (SGLT1 and SGLT2) inhibition activity.

 Please wait while we load your content...
Please wait while we load your content...