Benchmark thermochemistry of chloramines, bromamines, and bromochloramines: halogen oxidants stabilized by electron correlation†
Abstract
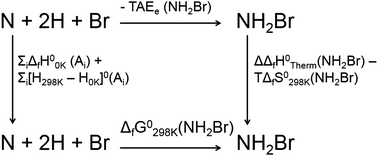
Chloramines, bromamines, and bromochloramines are halogen-containing oxidants that arise from the reaction of hypohalous acids with ammonia in water. Although relevant to both water disinfection chemistry and biochemistry, these molecules are difficult to study in the laboratory, and their thermochemical properties remain poorly established. We developed a benchmark level ab initio calculation protocol, termed TA14, adapted from the Weizmann theory and Feller–Peterson–Dixon approaches to determine the molecular structures and thermochemical properties of these compounds. We find that the halamine molecules are bound largely, and in some cases entirely, by electron correlation forces. This presumably explains their high reactivity as electrophilic oxidants. We provide computed heats of formation at 0 K (ΔfH00 K) and at 298 K (ΔfH0298 K) and Gibbs free energies of formation at 298 K (ΔfG0298 K) for the 9 inorganic chloramines, bromamines, bromochloramines in gas phase. Based on comparisons to previous theoretical and experimental data for a set of 11 small molecules containing N, O, H, Cl, and Br, we propose uncertainties ranging from 1 to 3 kJ mol−1 for computed thermodynamic properties of the halamines. Reported thermochemical data enable the determination of equilibrium constants for reactions involving halamines, opening possibilities for more quantitative studies of the chemistry of these poorly understood compounds.

 Please wait while we load your content...
Please wait while we load your content...