Photophysical properties of the intramolecular excited charge-transfer states of π-expanded styryl phenanthrimidazoles – effect of solvent polarity†
Abstract
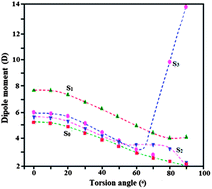
Solvent-dependent electronic structures of selected donor (D)–acceptor (A) phenanthrimidazole derivatives containing styryl as an electron acceptor fragment in fluorescent charge transfer (CT) states have been investigated. Radiative charge recombination [CT → S0] is discussed in terms of the Mulliken–Murrell model of the CT complexes and the Marcus theory of photoinduced electron transfer (ET). Solvatochromic effects on the fluorescence spectra indicate the CT character of the emitting singlet states and the analysis leads to the electron transfer in the Marcus inverted region. The fluorescence rate constants (kr) and transition dipole moments (M) indicate that the electronic coupling between the emitting CT state and the ground state is a governing factor of the radiative transitions. Large values of M indicate a nonorthogonal geometry (confirmed by XRD) of the donor and acceptor subunits in the fluorescent states. Emission spectra of N,N-dimethyl-4-(2-styryl-1H-phenanthro[9,10-d]imidazol-1-yl)benzenamine is of interest because of the existence of dual emitting states where a locally excited state is responsible for fluorescence in nonpolar solvents. In polar solvents fluorescence is from a twisted intramolecular charge transfer (TICT) state. Density functional theory (DFT) calculations support the formation of the TICT state. The twist of the –N(CH3)2 group and the change in its hybridization in the excited state develops a high dipole moment and thereby stabilizes it to give the TICT fluorescence in polar solvents.

 Please wait while we load your content...
Please wait while we load your content...