Solvent mediated thermodynamically favorable helical supramolecular self-assembly: recognition behavior towards achiral and chiral analytes†
Abstract
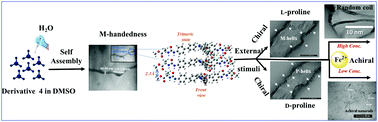
Herein, we successfully developed an entropically favored helical supramolecular self-assembly from a triphenylamine-based derivative 4 in a green solvent in order to mimic the structural transformations that occur during the self-assembly of proteins/peptides which may cause various neurodegenerative diseases. Its structural transformation from helical supramolecular self-assembly to a random coil and then achiral nanorods was studied by varying the concentration of achiral stimuli (i.e., Fe2+ ions). The driving force of this transformation is the strong binding affinity of chiral supramolecular assemblies and Fe2+ ions. Furthermore, the “metal-free” helical supramolecular self-assembly exhibited enantioselectivity for differentiating between L- and D-proline; this was achieved through a chiral stimuli-induced structural modulation methodology. Our evaluation of the effects of achiral/chiral stimuli is also novel.


 Please wait while we load your content...
Please wait while we load your content...