Recent advances in green multi-component reactions for heterocyclic compound construction
Xinling
Shen
a,
Gang
Hong
 ab and
Limin
Wang
ab and
Limin
Wang
 *a
*a
aKey Laboratory for Advanced Materials and Institute of Fine Chemicals, East China University of Science and Technology, 130 Meilong Road, Shanghai 200237, P. R. China. E-mail: wanglimin@ecust.edu.cn; Fax: +86-21-64253881; Tel: +86 -21-64253881
bDepartment of Chemistry, Roy and Diana Vagelos Laboratories, University of Pennsylvania, Philadelphia, Pennsylvania 19104-6323, USA
First published on 21st January 2025
Abstract
Multi-component reactions (MCRs) are processes in which three or more reactants are introduced into one pot to obtain the final product with high atom efficiency, and in recent years, these have become a key strategy for advancing more sustainable processes in modern synthetic communities and the pharmaceutical industry. Meanwhile, minimizing the use of solvents, catalysts, time, reagents, and waste is essential during green chemical synthesis to reduce cost and environmental impact. Heterocycles are ubiquitous and have thus prompted the development of numerous methods for their synthesis. Among various strategies, MCRs represent one of the most promising routes for the synthesis of heterocyclic moieties such as quinolines, quinazolines, pyrimidines and imidazoles, which are widely recognized in nature and clinical evaluation. To promote greener syntheses, a significant body of literature detailing the synthesis of these biologically important compounds via environmentally friendly MCRs has emerged. This review focused on the recent advances in the green approach to preparing heterocyclic compounds via MCRs. These green approaches included photoredox catalysis, electrochemical activation, catalyst-free methods, and the use of water as the sole green solvent, reported between 2018 and 2024, highlighting their strengths and limitations. The synthesis of different types of heterocycles via green MCRs was covered. The substrate scope, reaction conditions, yields and mechanisms were also examined and discussed.
1. Introduction
In recent years, there has been growing interest among synthetic organic chemists in the development of greener and efficient methods for the construction of complex organic molecules. Heterocyclic compounds, which are widely present in chemical entities and natural products, constitute a highly significant class of molecules. Products derived from multi-component reactions (MCRs) can be regarded as a synthetic hub for a wide variety of novel heterocyclic compounds. In general, MCRs involve three or more reactants being introduced into a single reaction vessel to form a new product.1 These types of reactions offer significant advantages, including simple operation, time/energy saving, and high atom utility, and are generally environmentally friendly.2 The Strecker synthesis of α-amino cyanides from ammonia, carbonyl compounds and hydrogen cyanide is generally regarded as the first MCR.3 Since then, the utility of MCRs has been consistently proven with their wide application in drug discovery, and some representative drugs produced through MCRs are listed in Fig. 1.4While MCRs are inherently sustainable, as most of the atoms in the starting substrates are retained in the final product,5 their sustainability can be further enhanced by incorporating green energy, catalysts and/or green solvents. Careful selection of energy sources and catalysts to enhance catalysis efficiency while adhering to the principles of green chemistry, along with modifications to the solvents used, can accelerate the shift toward eco-friendly chemistry. In addition to reducing the loading or even avoiding the use of catalysts, the employment of green energy sources, recyclable catalysts and/or water as the reaction solvent can have a positive impact.
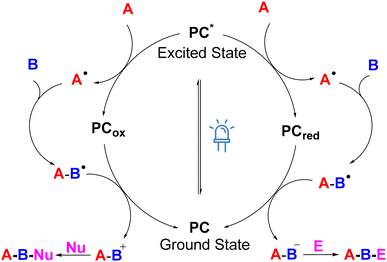
Recently, the broader applicability of MCRs has been reinforced by the renaissance of radical chemistry. In this regard, photo/electro-synthesis provides chemists with numerous innovative and versatile synthetic methodologies. The significant growth of research in organic photo- and electrochemistry over the past decades has established them as among the most competitive approaches in modern organic chemistry.6,7 The key characteristic of photo- and electrochemistry is the utilization of photons and electrons as energy sources. Both catalytic systems can produce highly reactive intermediates with exceptional selectivity and efficiency without the traditional use of hazardous chemicals. Furthermore, the use of these green energy sources can efficiently kick-start cascade reactions, especially in the frame of MCRs. In terms of photoredox-catalyzed MCRs, most examples discussed here primarily involve single-electron transfer (SET) processes. These begin with the photocatalyst being excited by visible light, followed by the single-electron oxidation or reduction of the radical source. The resulting radical intermediate typically undergoes an addition reaction with unsaturated bonds, such as alkenes, alkynes, or imines, forming a new radical intermediate. Then, a second single-electron oxidation/reduction of the radicals generates a formal positive/negative charge, which can further react with nucleophiles/electrophiles (Fig. 3). In electrocatalytic processes, the application of electric potential at the electrodes triggers SET events with the substrates in solutions. Under constant current conditions, the potential is variable and will adjust to allow current flow in the solution by generating redox events at both the electrodes. Under constant potential conditions, the potential is kept unchanged with the aid of a reference electrode (Fig. 4).
Besides the abovementioned photo/electrochemistry as a green energy source for MCRs, the replacement of conventional homogeneous catalysts with heterogeneous catalysts, which are easy to handle and usually recyclable, is another green approach to MCRs.8 Among these, zeolites are gaining increasing attention and are being increasingly used for producing value-added chemicals including those derived from biomass, with a clear trend towards high-value compounds.9 Zeolites have attractive properties such as tunable acidity and pore size and the possibility of inserting additional metals into their framework.10 In this regard, some reviews are available in the literature on zeolite-catalyzed heterocycle synthesis.11
Of course, if organic reactions can be successfully performed without any catalysts, they would provide even greener methods for the formation of organic compounds. Some common difficulties and limitations associated with catalyst-free reactions such as low selectivity, high temperature, longer reaction time and low yield can be overcome to some extent by (a) a careful choice of solvent, which can efficiently improve the product yield, (b) using appropriate active substrates, and (c) applying a suitable reaction strategy (e.g. microwave heating, ball milling, ultrasonication, reaction under high pressure, etc.).
Solvents are the most significant constituents in terms of quantity in organic reactions, and the wise choice of solvent species and reducing the use of petroleum derived organic solvents play a pivotal role in reducing the environmental impact. In fact, as R. A. Sheldon has clearly stated, it is widely acknowledged that “the best solvent is no solvent and if a solvent (dilute) is needed it should be preferably be water”.12 Longstanding efforts and interest in our group have focused on developing various MCRs as well as electrochemically induced reactions in the past decades.13 In this review, we outline recent applications of photoredox catalysis and electrochemical activation in the synthesis of heterocyclic frameworks via MCRs paying particular attention to catalyst-free MCRs and reactions performed in water (2018–2024) (Fig. 2).
2. Classification
For clarity and simplicity, the following discussion has been organized based on different green approaches toward MCRs: (i) photoredox-catalyzed MCRs, (ii) electrochemically activated MCRs, (iii) catalyst-free MCRs, and (iv) water as the reaction medium for MCRs.2.1 Photoredox-catalyzed MCRs
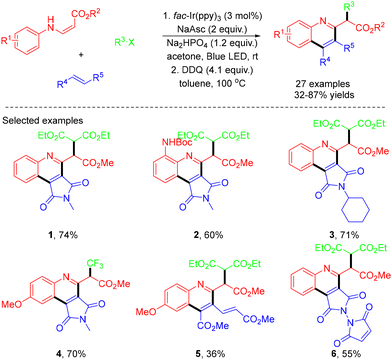
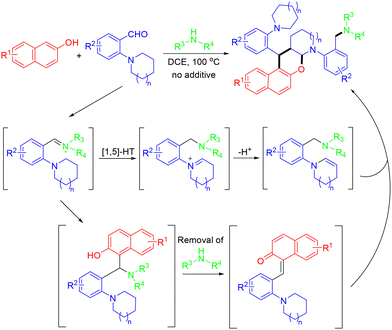
In 2018, Park and co-workers demonstrated visible-light photoredox-catalyzed three-component quinoline synthesis using fac-Ir(ppy)3 as the photocatalyst. This method, involving three consecutive radical-mediated bond formations, allowed chemoselective incorporation of coupling partners β-aminoacrylates, halides, and alkenes, leading to the formation of quinolines in 32–87% yields (Scheme 1).15
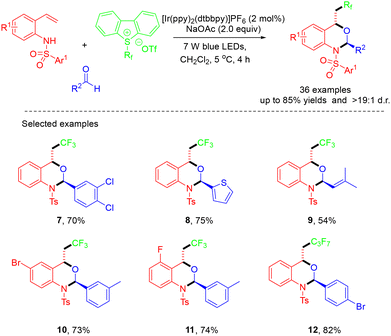
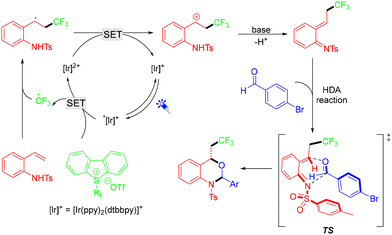
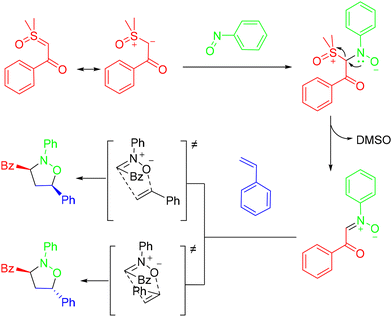
A visible-light-promoted, intermolecular hetero-Diels–Alder reaction of photogenerated aza-ortho-quinone methides with aldehydes under mild conditions was demonstrated by the Chen group (Scheme 2).16 This protocol featured a wide substrate scope, a simple reaction procedure, and excellent diastereoselectivity, providing practical access to valuable perfluoroalkylated dihydrobenzoxazines. According to the mechanism, the CF3 radical was generated through single-electron transfer (SET) reduction of the Umemoto reagent by the excited state *[Ir]+. Then, the electrophilic CF3 radical underwent addition to an alkene to afford a benzylic radical, which can be further oxidized by [Ir]2+ species to generate a benzylic cation. In the presence of the base NaOAc, the benzylic cation underwent facile deprotonation to afford the key intermediate aza-o-QM, which further reacted with aldehydes through a concerted HDA reaction pathway to deliver the product (Scheme 3).
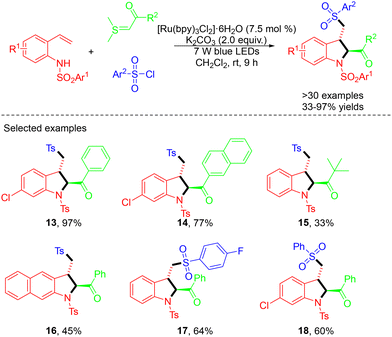
In 2020, Chen and co-workers demonstrated a visible-light-driven photoredox-catalyzed MCR of 2-vinylanilines, sulfonyl chlorides, and sulfur ylides. Here, the optimal reaction conditions were found to be: 1 equiv. of 2-vinylanilines, 3 equiv. of sulfonyl chlorides, and 3 equiv. of sulfur ylide in the presence of K2CO3 (2.0 equiv.) as a base and 7.5 mol% of [Ru(bpy)3Cl2]·6H2O as a photocatalyst in CH2Cl2 at room temperature under the irradiation of 7 W blue LEDs (Scheme 4).17
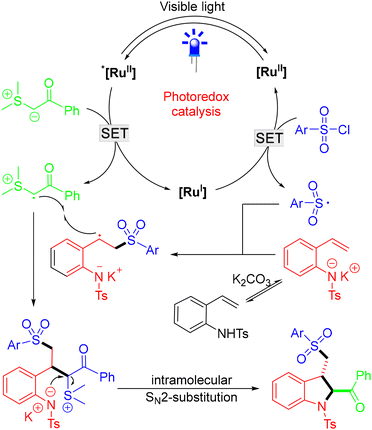
Based on control experiments, the author proposed that the reaction began with SET oxidation of the sulfur ylide by the excited state *[RuII], generating the radical of the sulfur ylide and [RuI]. Meanwhile, SET reduction of sulfonyl chloride by the [RuI] species produced the electrophilic sulfonyl radical and regenerated the ground-state photocatalyst [RuII]. Then, the sulfonyl radical attacked the alkene of the in situ formed 2-vinylaniline salt to afford the radical intermediate, which underwent radical–radical coupling with the sulfonyl radical and subsequent intramolecular SN2 substitution to form the final cyclized product (Scheme 5).
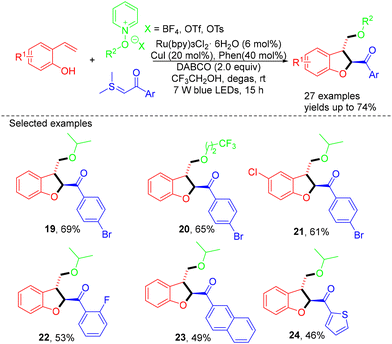
In 2021, the same group reported the synthesis of 2,3-dihydrobenzofuran through visible-light-driven Ru(bpy)3Cl2·6H2O-catalyzed MCRs of 2-vinyl phenols, N-alkoxypyridinium salts, and sulfur ylides (Scheme 6).18 The performance of the reaction relied on the utility of both sulfur ylides and N-alkoxypyridinium salts as radical precursors. This redox-neutral protocol demonstrated excellent functional group tolerance, readily accessible starting materials, and benign conditions, allowing for the preparation of a broad range of valuable 2,3-disubstituted dihydrobenzofurans.
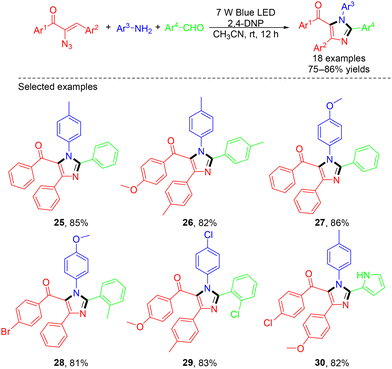
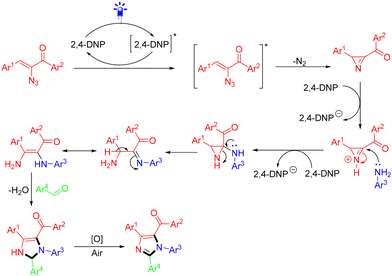
In 2023, Maurya et al. developed an efficient visible light-photocatalyzed one-pot, three-component synthesis of highly functionalized imidazoles (Scheme 7).20 In this work, the successful reaction of anilines, aldehydes, and α-keto vinyl azides to form imidazoles under 7 W blue LED light required the use of 2,4-dinitrophenol (2,4-DNP) as a photocatalyst. 2,4-DNP was excited by absorbing blue LED light. Then, it transferred the energy to α-keto vinyl azide, which released a molecule of dinitrogen to form a 2H-azirine intermediate. The 2H-azirine could get protonated by 2,4-DNP to form an intermediate, which was attacked by aniline to form aziridine. Subsequently, the lone pair electrons of the nitrogen atom in the aziridine led to ring opening, affording an imine intermediate, which was isomerized, followed by coupling with an aldehyde and further air oxidation to afford the final desired imidazole (Scheme 8).
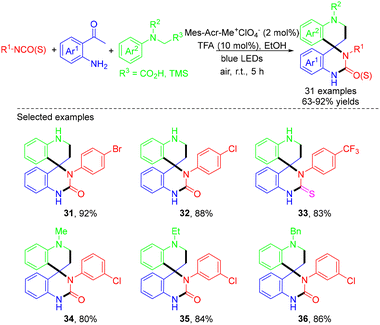
The Ma group presented a visible-light-induced, Mes-Acr-Me+ClO4−-catalyzed MCR for the formation of a wide variety of spiroquinzolin-2-(thi)ones from iso(thio)cyanates with 2-aminoacetophenones and α-amino acids or α-silyl amines in 2022.21 A wide variety of substrates were compatible with this reaction system, delivering the corresponding spiroquinazolin-2-(thi)ones in 63–92% yields (Scheme 9). However, the reaction with 2-isocyanato-2-methylpropane failed to afford the desired product due to steric effects. In the same year, Kendrekar et al. demonstrated four-component green synthesis of biologically active 1,8-naphthyridines through the reaction of various aromatic aldehydes, malononitrile, 1,6-dimethylpyridin-2(1H)-one, and the corresponding aniline catalyzed by Mes-Acr-Me+ClO4− under visible light originating from a 24 W blue LED with wavelengths of 450–460 nm.22
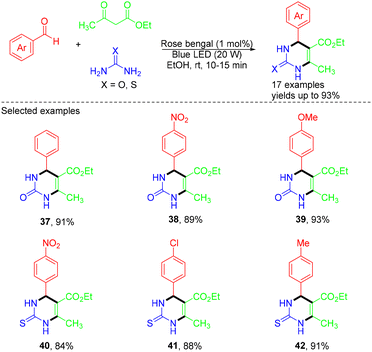
In 2024, an efficient method for synthesizing 3,4-dihydropyrimidin-2(1H)-ones/thiones in EtOH with the combination of visible light and rose bengal, a non-toxic organic dye, as a photoredox catalyst was reported by the Singh group (Scheme 10).23 In the catalytic cycle, rose bengal (RB) was excited from the ground state to the excited state (RB*) under visible light irradiation. Next, the energy was transferred from RB* to the enol form of ethyl acetoacetate, generating the cation radical intermediate and RB˙− through SET. The electron transfer activity of the RB˙− and aromatic aldehydes led to the revival of the ground state of RB and radical anion intermediate. Subsequently, a reactive iminium intermediate was generated by adding this radical anion to urea/thiourea. This intermediate was further attacked by the cation radical intermediate, followed by cyclization and dehydration to produce the final product (Scheme 11).
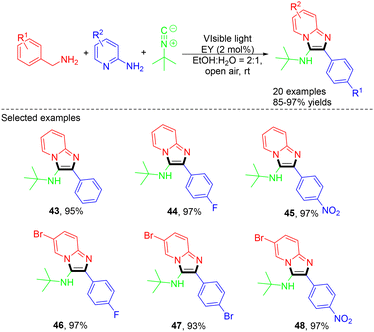
In 2020, the Singh group developed an environmentally friendly approach for the synthesis of the biologically important aminoimidazopyridine through the MCR of benzylamine, 2-aminopyridine, and t-butyl isocyanide under visible light with eosin Y as an organic photocatalyst (Scheme 12).24 This approach is straightforward, environmentally friendly, and free of additives and metals, while also demonstrating excellent compatibility with both electron-donating and electron-withdrawing functional groups. In 2022, Farzaneh Mohamadpour described a method utilizing Knoevenagel–Michael cyclocondensation with malononitrile, aryl aldehydes, and resorcinol, serving as a sustainable MCR for synthesizing 2-amino-4H-chromene scaffolds using Na2EosinY as a photocatalyst.25 Several compounds were synthesized with yields ranging from 85% to 96%.
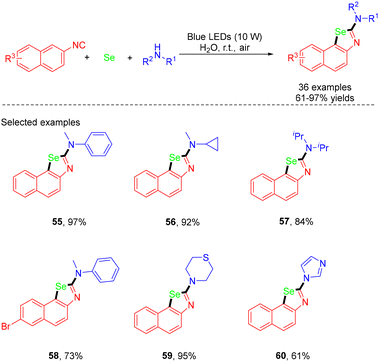
In 2023, the He group reported an unprecedented eco-friendly approach for the visible-light photocatalytic MCRs of naphthoselenazol-2-amines from isocyanonaphthalenes, selenium powder and secondary amines with ambient air as the clean oxidant in water and under exogenous photosensitizer- and additive-free conditions (Scheme 15).27 A wide range of functionalized naphthoselenazol-2-amines with various valuable functional groups were obtained. The use of benzonitrile in place of 2-isocyanonaphthalene did not yield the target product. Similarly, substituting N-methylaniline with a primary amine or amide also failed to produce the desired product. The same group developed electrochemical MCRs for the synthesis of selenazol-2-amines in 2024.28
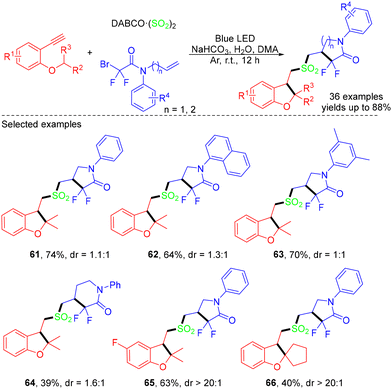
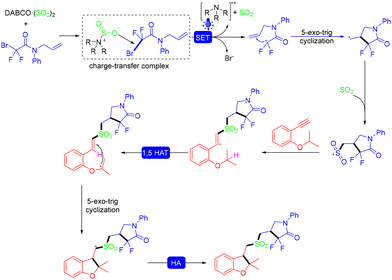
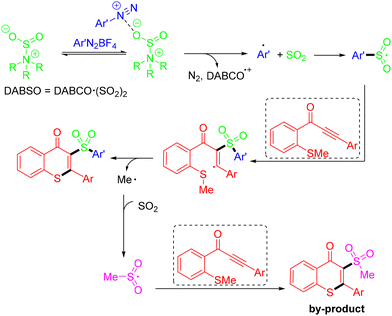
In 2022, the Yu group developed the visible light-induced photocatalyst-free MCRs of N-allylbromodifluoroacetamides and terminal alkynes, which involve the insertion of SO2via the formation of a charge-transfer complex and an intramolecular 1,5-hydrogen atom transfer. Four new bonds including two C–S bonds were formed in the reaction. Unfortunately, no desired products were obtained when cyclopentyl and pyridyl N-allylbromodifluoroacetamides were tested (Scheme 16).29 In the mechanistic study, the combination of N-allylbromodifluoroacetamides and DABCO·(SO2)2 generated a charge-transfer complex, which underwent a visible light-induced SET process, providing the difluoroalkyl radical, a tertiary amino radical cation, and sulfur dioxide. Then, rapid 5-exo-trig radical cyclization generated the cyclic radical intermediate, which was then trapped by sulfur dioxide to produce a difluoroamidosulfonyl radical. The vinyl radical was formed by the difluoroamidosulfonyl radical attacking the substrate 1-ethynyl-2-isopropoxybenzene, which sequentially underwent an intramolecular 1,5-HAT reaction and another 5-exo-trig radical cyclization process to afford the cyclic radical intermediate. Finally, hydrogen abstraction from water or the solvent to the intermediate would yield the corresponding product (Scheme 17).
2.2 Electrochemically activated MCRs
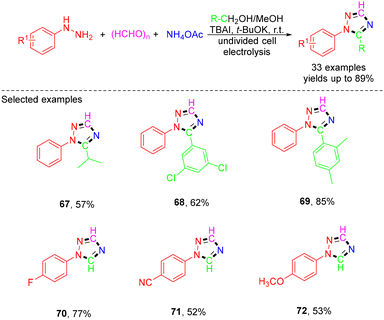
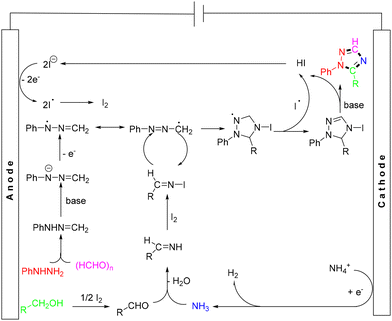
In 2018, a novel electrochemical route was developed by Yuan et al. for the synthesis of 1,2,4-triazoles from four components aryl hydrazines, paraformaldehyde, NH4OAc, and alcohols (Scheme 18).31 This process featured the avoidance of the use of strong oxidants and transition-metal catalysts, affording a wide array of 1,2,4-triazole derivatives in good to high yields. The mechanism for this reaction was proposed as follows: initially, I− ions were electro-oxidized at the anode to generate a reactive iodide radical or I2. Meanwhile, phenylhydrazine reacted with paraformaldehyde to produce the intermediate 1-methylene-2-phenylhydrazine, which, in the presence of tBuOK, could form the 1-methylene-2-phenylhydrazine anion. This anion was further electrochemically oxidized to yield a radical, which was then converted to a resonance structural radical. On the other hand, the alcohol can be oxidized to the corresponding aldehyde in the presence of I2. The aldehyde then reacted with NH3 produced by the electroreduction of NH4+ ions at the cathode to form an aldimine intermediate, which subsequently reacted with I2 to generate an N-iodo aldimine intermediate. The intermolecular radical cycloaddition of the radical with N-iodo aldimine formed a cyclic radical. Then, the end product was generated by deprotonation of the cyclic radical and aromatization with the aid of the iodide radical and tBuOK (Scheme 19).
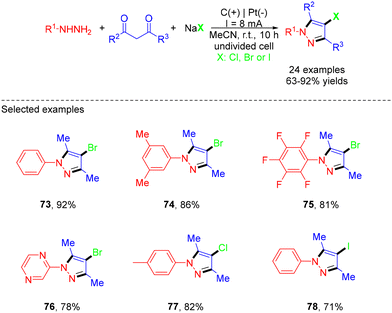
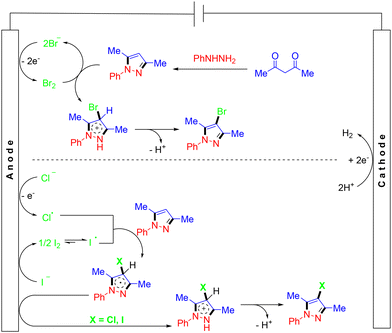
In 2022, He et al. demonstrated electrocatalytic multicomponent synthesis of 4-chloro/bromo/iodopyrazoles from hydrazines, acetylacetones and sodium halides under conditions free of chemical oxidants and external electrolytes (Scheme 20).32 A variety of 4-haloyrazoles were obtained, which could serve as handles for future late-stage synthesis in medicinal chemistry. However, no reaction happened when KF, KSCN or KSeCN replaced NaBr. In the mechanistic study, they proposed that sodium halides played a dual role as a halogenation reagent and a supporting electrolyte. The reaction was initiated by the formation of a pyrazole intermediate through the reaction of phenylhydrazine with acetylacetone. The anodic oxidation of a bromide ion generated Br2, which could react with pyrazole to afford the cation intermediate along with a bromide ion. Finally, the corresponding pyrazole product was generated by the deprotonation of the cation intermediate and the restoration of the aromatic system. For the formation of chloro- or iodo-substituted pyrazole, the chlorine/iodine radical was first generated at the anode. Subsequently, the coupling of the chlorine/iodine radical with pyrazole produced the radical intermediate, which then underwent anodic oxidation to form the pyrazole cation intermediate. Finally, deprotonation and re-aromatization resulted in the formation of the desired products (Scheme 21).
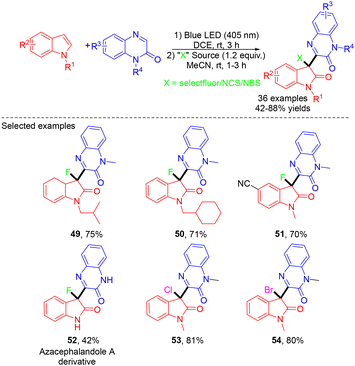
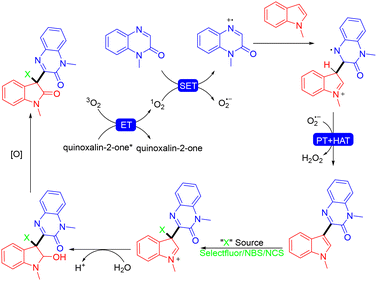
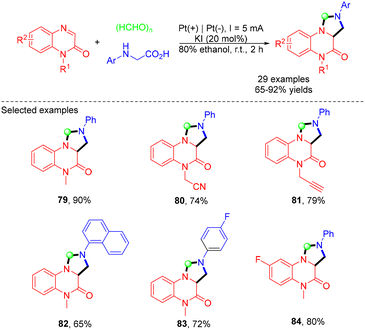
In 2023, the same group reported an electrochemical approach for the synthesis of tetrahydroimidazo[1,5-a]quinoxalin-4(5H)-ones via the MCRs of quinoxalin-2(1H)-ones, N-arylglycines and paraformaldehyde (Scheme 22).33 A wide range of imidazo[1,5-a]quinoxalinone derivatives with various valuable functional groups were obtained in good to excellent yields from easily accessible starting materials. Notably, this work featured the use of paraformaldehyde as the C1 synthon, not only increasing the atom economy but also enhancing the practicability and environmental friendliness. In the mechanistic study, the authors proposed that EtOH played a dual role as both a catalyst and a reaction solvent, which simplified the reaction system. Using a similar strategy, the same group achieved electrochemical MCRs of various imidazolidine-fused sulfamidates.34
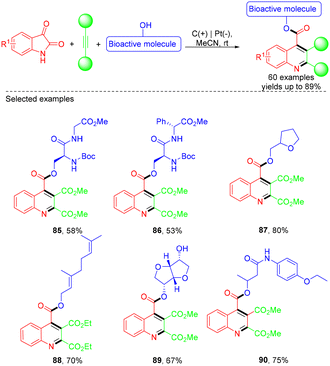
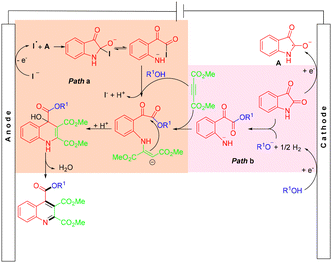
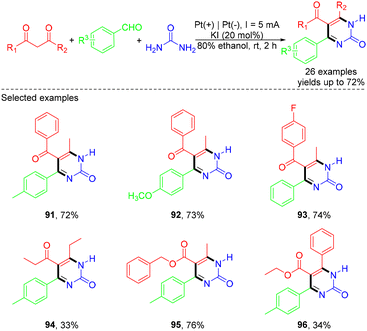
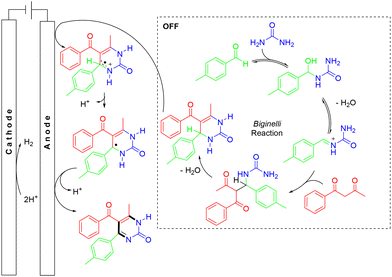
In 2019, the Wu group developed a green and simple electrochemical approach to synthesize pyrimidin-2(1H)-ones via Biginelli condensation and direct electrooxidation (Scheme 25).36 The electrooxidation approach exhibited excellent substrate compatibility, providing various pyrimidin-2(1H)-ones in moderate to good yields. However, aliphatic aldehydes were incompatible with the ‘one-pot’ reaction conditions. According to the proposed experiments, the first step, under electric current off conditions, involved the Biginelli reaction, where the condensation between the urea and aldehyde formed an iminium intermediate, which could act as an electrophile for the following nucleophilic addition of β-dicarbonyls. The resulting intermediate then underwent a condensation reaction with the other free NH2 of urea to form the cyclized product. Subsequently, under the electrolysis conditions, the intermediate lost an electron at the anode to generate a radical cation, followed by deprotonation of the radical cation and loss of another electron, leading to the formation of the desired product. On the other hand, H+ or EtOH was reduced to hydrogen on the cathode (Scheme 26).
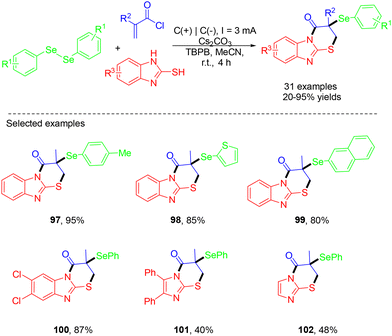
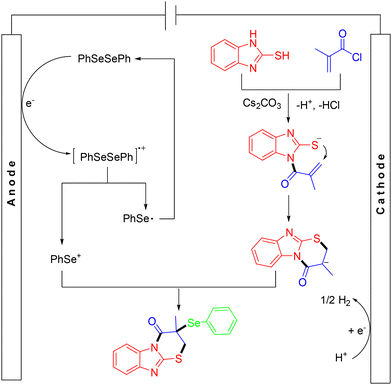
In 2024, the Liu group demonstrated a green method for the efficient synthesis of an array of selenyl imidazo[2,1-b]thiazinones from acryloyl chlorides, diselenides and 2-mercaptobenzimidazoles via electrochemical MCRs (Scheme 27).37 This methodology offered the advantages of good functional group tolerance, simple reaction conditions, and high step economy. In the mechanism, diselenide was first oxidized at the anode to form the cationic radical intermediate, which dissociated into a selenium cation and a selenium radical. Diselenide was regenerated through auto-coupling of the selenium radical. In the presence of a base, the reaction between 2-mercaptobenzimidazole and methacryloyl chloride resulted in the formation of an amide intermediate, which then underwent intramolecular cyclization to construct a six-membered ring. Finally, this intermediate was captured by the selenium cation to afford the target product (Scheme 28).
2.3 Catalyst-free MCRs
In organic synthesis, a catalyst is often needed to accelerate the chemical reaction, thereby increasing the yield of the product. Unfortunately, in most cases, catalysts are toxic, costly and environmental pollutants. Besides, in many cases, especially in homogeneous catalysis, separating or removing the catalyst after the reaction is complete is difficult, time-consuming, and remains a major challenge in large-scale synthesis.38 If organic reactions can be successfully performed without catalysts, then they would provide greener and more economical routes for the formation of organic compounds. The advantages of catalyst-free reactions include being less sensitive to air and moisture compared to many metal/non-metal catalyst-based reactions, producing fewer or no byproducts due to the minimized side reactions, enabling simple purification of products, and having an easy operating procedure since there is no need for catalyst weighing, screening or removal (Fig. 5). Meanwhile, the term “catalyst-free” does not always imply that the reaction is not catalyzed. In many cases, it has been reported that either solvent, oxygen, air or the substrate itself can catalyze the reactions. Self-catalyzed reactions are also considered catalyst-free.39Furthermore, the author conducted molecular docking studies of the compounds, revealing that the synthesized compounds showed good binding affinities with Jack bean urease.
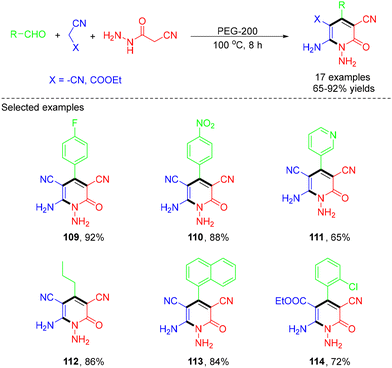
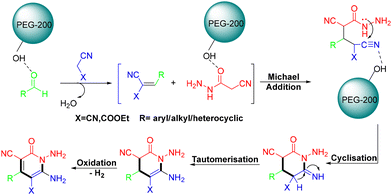
In 2023, the Ghosh group demonstrated the use of PEG-200 to mediate MCRs for the synthesis of a wide variety of highly functionalized 3-cyano-2-pyridones, through MCRs of aldehydes, cyanoacetohydrazide and malononitrile/ethyl cyanoacetate (Scheme 30).41 The utilization of the biodegradable greener solvent PEG-200 without a catalyst, easily accessible synthons, mild reaction conditions and easy purification of the desired products are the foremost advantages of this protocol. According to the mechanism, first, PEG-200 coordinated with an aldehyde to enhance its electrophilic character, after which malononitrile or ethyl cyanoacetate quickly reacted with the aldehyde to form the Knoevenagel condensation product. Subsequently, the intermediate then reacted with the third reactant cyanoacetohydrazide, via Michael addition, followed by cyclisation, tautomerization and oxidation to generate the target product (Scheme 31).
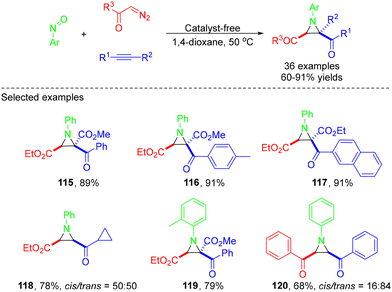
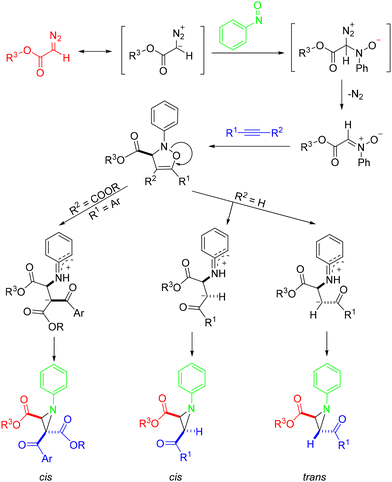
In 2020, the Wei group developed a straightforward, catalyst-free approach for the synthesis of multisubstituted 1-arylaziridine-2-carboxylates via one-pot MCRs of α-diazoesters, nitrosoarenes, and alkynes (Scheme 32).42 An array of highly substituted aziridines could be afforded in up to 91% yields under mild conditions. Unfortunately, no corresponding products were obtained with disubstituted diazo compounds such as dimethyl 2-diazomalonate. The process starts with the reaction of α-diazoester with nitrosobenzene to afford an intermediate, which further releases N2 to generate the nitrone intermediate. Then, this nitrone could react with an alkyne via [3 + 2] cycloaddition to afford an isooxazoline. Subsequently, Baldwin rearrangement of the isooxazoline produced the desired cis- or trans-product depending on the functional group attached to the alkyne (Scheme 33).
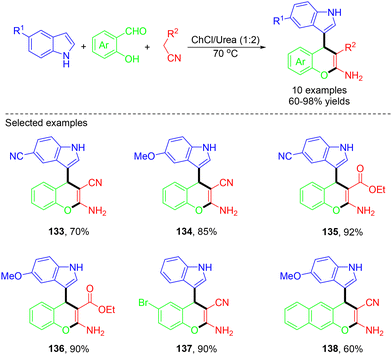
Chromene derivatives, also referred to as benzopyrans, are an essential class of heterocycles. They serve as key components in various natural products used as photoactive compounds and pigments while exhibiting a wide array of pharmaceutical activities, including antiviral, anti-anaphylactic, diuretic, anticoagulant, spasmolytic, and sex pheromone properties. Some structures of some important chromene derivatives are displayed in Fig. 6.44 In 2023, the Ali group demonstrated facile catalyst-free one-pot MCRs of indole-centered 4H-chromenes from readily available indole derivatives, salicylaldehyde, 2-hydroxy-1-naphthaldehyde, malononitrile, and ethyl cyanoacetate in a deep eutectic solvent (DES) (Scheme 36).45 The current procedure offers several advantages, such as high yields, mild reaction conditions, relatively shorter reaction times, straightforward workup, and the recyclability of the reaction medium.
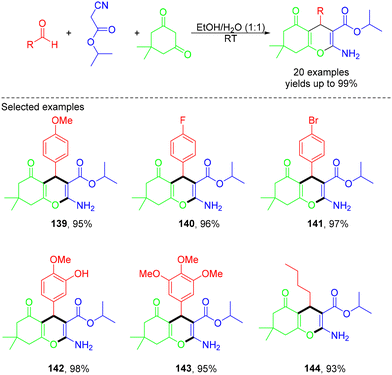
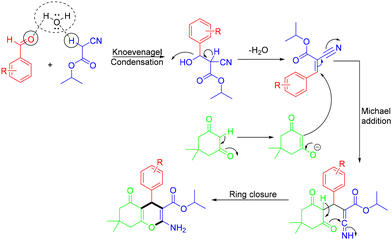
In 2020, the Jonnalagadda group demonstrated green catalyst-free synthesis of 2-amino-4H-chromene derivatives via MCRs of aldehydes with isopropyl cyanoacetate and 5,5-dimethyl-1,3-cyclohexanedione in aqueous ethanol under ultrasound irradiation (Scheme 37).46 The desired products were obtained in excellent yields (95–99%) within short reaction times (5–15 minutes). This method avoided the use of expensive catalysts, organic solvents, and tedious column chromatography purification. This mechanism is illustrated in Scheme 38. Initially, under ultrasound irradiation, the simultaneous activation of the carbonyl compound and the active methylene compound substrates occurred via hydrogen bond formation with the water medium, enhancing the electrophilicity of the carbonyl compound and the nucleophilicity of the active methylene carbon atom. The nucleophile then underwent intermolecular nucleophilic attack on the carbonyl carbon of the aldehyde, yielding the Knoevenagel intermediate with the removal of a water molecular. Then, this intermediate underwent Michael addition with enolate dimedone, leading to the formation of a Michael adduct. Finally, this adduct underwent ring closure, generating the desired product.
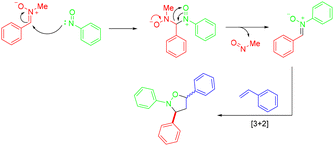
In 2021, the Li group utilized N-alkyl nitrones as the starting materials to synthesize various N-aryl isoxazolidines via catalyst-free MCRs with olefins and nitrosoarenes (Scheme 41).48 This methodology featured a broad substrate scope, good functional group tolerance, and mild reaction conditions. Unfortunately, aliphatic aldehyde-based N-methyl nitrones such as (Z)-N-(2-chloroethylidene)methenamine oxide were not compatible with the current protocol. A plausible mechanism was proposed based on the control experiment. The attack of nitrosobenzene on the carbon atom of (Z)-N-benzylidenemethanamine oxide generated an intermediate, which could be further transformed into an N-aryl nitrone intermediate with the expulsion of nitrosomethane. Finally, the products were constructed via the [3 + 2] cycloaddition with styrene (Scheme 42).
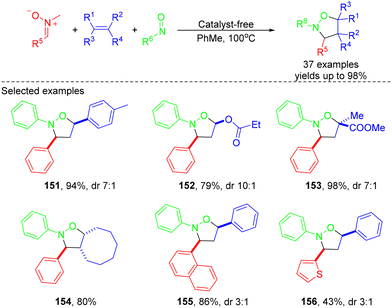
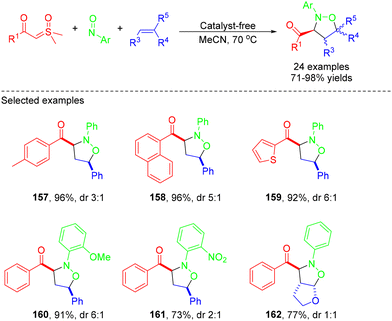
In 2021, the same group employed a similar strategy to construct various keto-substituted isoxazolidines via one-pot MCRs of stable sulfoxonium ylides, nitrosoarenes and olefins (Scheme 43).49 A variety of α-carbonyl sulfoxonium ylides featuring diverse functional groups such as aryl, heteroaryl and alkyl ones could be effectively utilized in this reaction. 71–98% yields of the corresponding products were obtained without a catalyst. The reaction mechanism was explained on the basis of previous reports and control experiments (Scheme 44).
2.4 Water as a reaction medium for MCRs
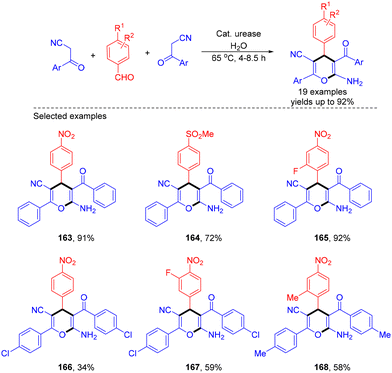
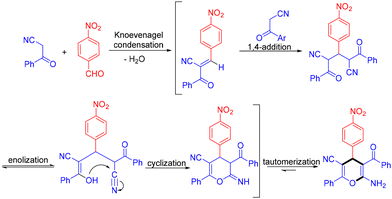
Solvents are generally essential in organic reactions to expedite mass and heat transfer processes, help with separations and purifications, and work as cleaning agents for industrial facilities. Nevertheless, to reduce the production cost and environmental impact, there is a need to minimize the generation of residues or waste resulting from organic solvents, and in this context, replacing these harmful ingredients with more environmentally friendly reaction media is a critically important target for the modern synthetic communuity.50 As a result, the interest in using water as a reaction medium in synthetic chemistry has experienced explosive growth in recent years.51 Water, the most abundant molecule on Earth, offers several advantages, including its unique redox stability and non-flammable, non-toxic, and non-hazardous nature. Additionally, using water as a solvent can sometimes promote alternative reactivity pathways or enhance selectivity. Moreover, the relatively high lipophilicity of most organic compounds simplifies product separation at the end of the reaction, streamlining work-up and isolation processes while reducing waste generation.In 2024, Beifuss et al. demonstrated an efficient urease-catalyzed approach for the synthesis of highly substituted 6-amino-4H-pyran-3-carbonitriles in water via a three-component reaction between one molecule of an aromatic aldehyde and two molecules of an arylacetonitrile (Scheme 45).52 The optimal results were realized when the reactions were conducted using 1296 units of urease as the catalyst, with water as the sole green solvent. The following possible reaction mechanisms were proposed. The reaction sequence started with Knoevenagel condensation between 4-nitrobenzaldehyde and benzoylacetonitrile to afford the corresponding Knoevenagel intermediate, followed by 1,4-addition of a second molecule of benzoylacetonitrile. Then, the resulting intermediate underwent enolization, intramolecular O-cyclization, and tautomerization to afford the final product 6-amino-4H-pyran-3-carbonitrile (Scheme 46).
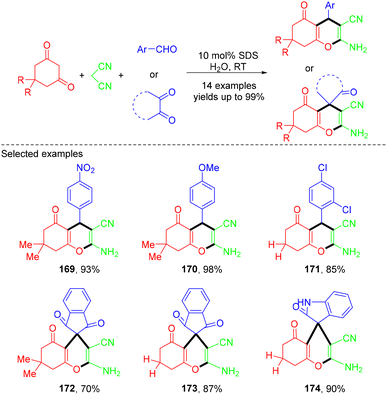
In 2023, the Jaitak group developed a one-pot three-component synthesis of substituted tetrahydrobenzo[b]pyran derivatives from MCRs of aromatic aldehydes, malononitrile and dimedone or 1,3-cyclohexanedione using sodium dodecyl sulphate as an efficient surfactant-type catalyst in water (Scheme 47).53 The synthesis of substituted spiropyrans was also achieved starting from ninhydrin/isatins, malononitrile and dimedone or 1,3-cyclohexanedione. All the reactions could be completed within a short reaction time (2.5 h), affording the desired products in good to excellent yields. According to the mechanism, the transformation is proposed to occur within the SDS hydrophobic interior via a domino Knoevenagel condensation/Michael addition/intramolecular cyclization sequence, providing a practical and green approach to tetrahydrobenzo[b]pyrans.
In 2022, the Shen group established a catalyst-free protocol for synthesizing indeno[1,2-b][1,6]naphthyridine-1,10(2H)-dione derivatives with good to excellent yields by microwave-assisted MCRs of 4-aminopyridin-2(1H)-ones, aldehydes and 1H-indene-1,3(2H)-dione in water (Scheme 48).54 Notably, up to four new bonds were formed, accompanied by generating water as the sole byproduct in this one-pot protocol. However, the reaction did not yield the desired product when aldehydes with electron-donating groups like methyl, methoxy, or isopropyl were used. The proposed mechanism for the microwave-assisted reaction is illustrated in Scheme 49. At the beginning, the nucleophilic addition between 1H-indene-1,3(2H)-dione and aldehydes produced an intermediate, which then underwent further transformation to form the alkene intermediate with removal of H2O, which further underwent Michael addition, intramolecular cyclization, and elimination of H2O to produce the desired product.
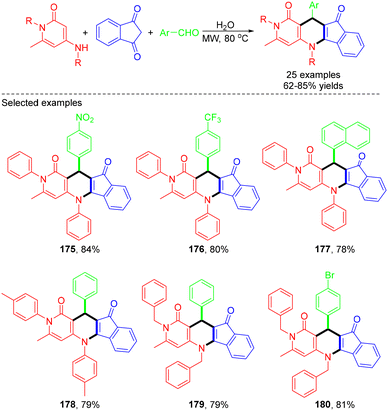 | ||
| Scheme 48 Synthesis of substituted indeno[1,2-b][1,6]naphthyridine-1,10(2H)-dione derivatives via microwave-assisted MCRs in water. | ||
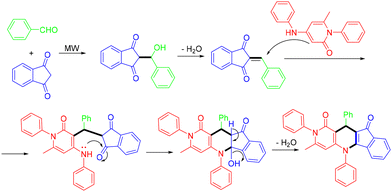 | ||
| Scheme 49 Proposed mechanism for the formation of indeno[1,2-b][1,6]naphthyridine-1,10(2H)-dione derivatives. | ||
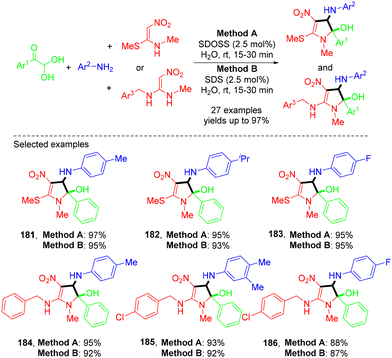
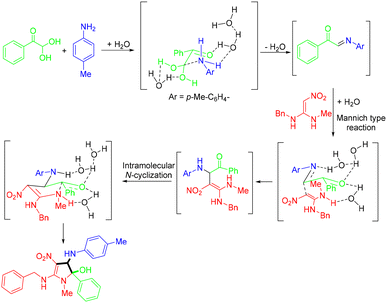
In 2024, the Seth group demonstrated a green strategy for the synthesis of substituted 2,3-dihydro-1H-pyrrol-2-ols “in-water” at room temperature (Scheme 50).55 The authors pointed out that water played an indispensable role in the presence of catalytic surfactants sodium dioctylsulfosuccinate and sodium dodecyl sulfate to facilitate the transformation. The reaction followed the mechanism presented in Scheme 51 through a sequence of imine formation and Mannich-type reactions, followed by intramolecular N-cyclization, and could deliver the corresponding products in good to excellent yields.
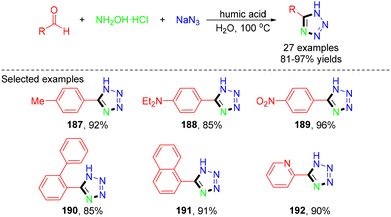
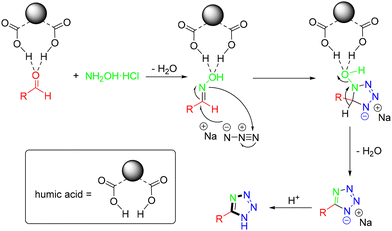
In 2020, the Wang group described a simple and facile synthesis of 5-substituted 1H-tetrazoles from one-pot MCRs of aldehydes, hydroxyamine hydrochloride and sodium azide using humic acid as a recyclable catalyst in water (Scheme 52).56 The strength of this methodology included the use of an easily accessible catalyst, water as the sole solvent, a simple experimental procedure, and high yields. In the mechanistic study, the carbonyl group of the aldehyde was activated by humic acid, followed by the nucleophilic attack of the nitrogen atom of hydroxylamine, providing aldehyde oxime. Then, the coordination of the protic acids with aldehyde oxime activated the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond, forming the intermediate for the subsequent nucleophilic addition of NaN3via a [3 + 2] cycloaddition mechanism. Finally, the elimination of water and acidic hydrolysis led to the formation of the target 1H-tetrazole (Scheme 53).
N bond, forming the intermediate for the subsequent nucleophilic addition of NaN3via a [3 + 2] cycloaddition mechanism. Finally, the elimination of water and acidic hydrolysis led to the formation of the target 1H-tetrazole (Scheme 53).
3. Conclusions
In summary, this article summarizes the latest findings (2018–2024) on the synthesis of heterocycles via green, environmentally friendly multi-component reactions, viz. (i) photoredox-catalyzed MCRs, (ii) electrochemically promoted MCRs, (iii) catalyst-free MCRs, and (iv) water as a reaction medium. Given the broad range of applications of heterocycles in natural products, agrochemicals, and pharmaceuticals, there has been an increase in articles published recently on the synthesis of these compounds including quinolines, dihydrobenzoxazines, indolines, dihydrobenzofurans, etc. Multicomponent reactions in general provide more sustainable methods to access highly functionalized heterocyclic compounds. Promising and fascinating results were found in many cases, for example, (i) synthesis of various dihydrobenzoxazines via visible-light-driven MCRs, (ii) electrosynthesis of diverse 4-halopyrazoles via MCRs, (iii) synthesis of spiroindolines via catalyst-free MCRs, and (iv) synthesis of substituted tetrahydrobenzo[b]pyrans via SDS-catalyzed MCRs in water. However, there are still some challenges that remain to be resolved in terms of this chemistry. For instance, achieving good enantioselectivities in the green MCRs that lead to compounds having stereogenic centers is still challenging. Furthermore, developing new green MCRs involving more than three components holds great potential and value for the chemistry community, although these higher component reactions have their own shortcomings due to the inherent difficulty in navigating the reaction in the right direction. Finally, once the synthetic method for a certain type of heterocycle has been developed via green MCRs, their application to target-oriented medicinal chemistry will surely promote their widespread acceptance.Author contributions
X. Shen: writing – original draft, investigation, and data curation. G. Hong: writing – review, reference collection, and investigation. L. Wang: writing – review & editing, supervision, outlining, and funding acquisition.Data availability
No new data were generated as this is a review paper.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (21772039, 21272069) and the Sinopec Seeding Program (ZC0607-0869), and we gratefully acknowledge financial support from the Central Universities and Science and Technology Commission of Shanghai Municipality (21DZ2305000) and the Fundamental Research Funds for the Central Universities (SLA13223001).References
- (a) A. Dömling, W. Wang and K. Wang, Chem. Rev., 2012, 112, 3083 CrossRef PubMed; (b) I. Ugi, A. Dömling and W. Hörl, Endeavour, 1994, 18, 115 CrossRef CAS; (c) C. Hulme and V. Gore, Curr. Med. Chem., 2003, 10, 51 CrossRef CAS PubMed; (d) R. W. Armstrong, A. P. Combs, P. A. Tempest, S. D. Brown and T. A. Keating, Acc. Chem. Res., 1996, 29, 123 CrossRef CAS.
- R. C. Cioc, E. Ruijter and R. V. A. Orru, Green Chem., 2014, 16, 2958 RSC.
- A. Strecker, Ann. Chem., 1850, 75, 27 CrossRef.
- (a) H. Wehlan, J. Oehme, A. Schäfer and K. Rossen, Org. Process Res. Dev., 2015, 19, 1980 CrossRef CAS; (b) C. Kalinski, H. Lemoine, J. Schmidt, C. Burdack, J. Kolb, M. Umkehrer and G. Ross, Synthesis, 2008, 4007 CrossRef CAS; (c) H. Liu, S. William, E. Herdtweck, S. Botros and A. Dömling, Chem. Biol. Drug Des., 2012, 79, 470 CrossRef CAS PubMed; (d) E. E. Wyatt, W. R. J. D. Galloway, G. L. Thomas, M. Welch, O. Loiseleur, A. T. Plowright and D. R. Spring, Chem. Commun., 2008, 40, 4962 RSC.
- A. Domling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3168 CrossRef CAS PubMed.
- selected reviews on photoredox catalysis, see: (a) L. Revathi, L. Ravindar, W.-Y. Fang, K. P. Rakesh and H.-L. Qin, Adv. Synth. Catal., 2018, 360, 4652 CrossRef CAS; (b) C. Zhu, H. Yue, L. Chu and M. Rueping, Chem. Sci., 2020, 11, 4051 RSC; (c) J. K. Matsui, S. B. Lang, D. R. Heitz and G. A. Molander, ACS Catal., 2017, 7, 2563 CrossRef CAS PubMed; (d) A. Kommoju, K. Snehita, K. Sowjanya, S. B. Mukkamala and K. Padala, Chem. Commun., 2024, 60, 8946 RSC.
- selected reviews on electrochemical catalysis, see: (a) Z. Yang, W. Shi, H. Alhumade, H. Yi and A.-W. Lei, Nat. Synth., 2023, 2, 217 CrossRef CAS; (b) K. Liu, C. Song and A.-W. Lei, Org. Biomol. Chem., 2018, 16, 2375 RSC; (c) M. D. Karkas, Chem. Soc. Rev., 2018, 47, 5786 RSC; (d) Y. Wang, S. Dana, H. Long, Y. Xu, Y. Li, N. Kaplaneris and L. Ackermann, Chem. Rev., 2023, 123, 11269 CrossRef CAS PubMed.
- S. Chassaing, V. Bénéteau, B. Louis and P. Pale, Curr. Org. Chem., 2017, 21, 779 CrossRef CAS.
- R. A. Sheldon, Green Chem., 2014, 16, 950 RSC.
- (a) T. Ennaert, J. V. Aelst, J. Dijkmans, R. D. Clercq, W. Schutyser, M. Dusselier and D. Verboekend, Chem. Soc. Rev., 2016, 45, 584 RSC; (b) F. S. Xiao and X. Meng, Zeolites in sustainable chemistry Heidelberg, Springer, 2016 CrossRef; (c) R. Srivastava, Catal. Today, 2018, 309, 172 CrossRef CAS.
- (a) M. J. Climent, A. Corma and S. Iborra, RSC Adv., 2012, 2, 16 RSC; (b) S. Chassaing, V. Beneteau and P. Pale, Curr. Opin. Green Sustainable Chem., 2018, 10, 35 CrossRef; (c) G. Bosica and R. Abdilla, Catalysts, 2022, 12, 725 CrossRef CAS; (d) G.-S.-C. Lima, N.-M. Moreira, M.-W. Paixão and A.-G. Corrêa, Curr. Opin. Green Sustainable Chem., 2019, 15, 7 CrossRef.
- R. A. Sheldon, J. Mol. Catal. A: Chem., 1996, 107, 75 CrossRef CAS.
- (a) J. Chen, Z.-C. Tang, G. Hong, Z. Zhou, T. Huang and L.-M. Wang, Tetrahedron Lett., 2024, 144, 155138 CrossRef CAS; (b) L.-M. Wang, J.-J. Xia, F. Qin, C.-T. Qian and J. Sun, Synthesis, 2003, 1241 CrossRef CAS; (c) L.-M. Wang, C.-T. Qian, H. Qian and Y. Ma, Synth. Commun., 2003, 33, 1459 CrossRef CAS; (d) J. Tang, L.-M. Wang, Y. Yao, L. Zhang and W. Wang, Tetrahedron Lett., 2011, 52, 509 CrossRef CAS; (e) Z. Tang, G. Hong, J. Chen, T. Huang, Z. Zhou and L.-M. Wang, Green Chem., 2023, 25, 9705 RSC; (f) Z. Tang, G. Hong, S. Sun and L.-M. Wang, Chem. – Asian J., 2023, 18, e202300001 CrossRef CAS PubMed.
- (a) P. Yadav, A. A. Varma, A. J. Punnya and P. Gopinath, Asian J. Org. Chem., 2022, 11, e202200390 CrossRef CAS; (b) S. Ariya, M. Neetha and G. Anilkumar, Curr. Catal., 2023, 12, 17 Search PubMed.
- J.-H. Choi and C.-M. Park, Adv. Synth. Catal., 2018, 360, 3553 CrossRef CAS.
- D. Liang, L. Rao, C. Xiao and J.-R. Chen, Org. Lett., 2019, 21, 8783 CrossRef CAS PubMed.
- M. M. D. Pramanik, F. Yuan, D.-M. Yan, W.-J. Xiao and J.-R. Chen, Org. Lett., 2020, 22, 2639 CrossRef CAS PubMed.
- F. Yuan, D.-M. Yan, P.-P. Gao, D.-Q. Shi, W.-J. Xiao and J.-R. Chen, ChemCatChem, 2021, 13, 543 CrossRef CAS.
- (a) M. Neumann, S. Füldner, B. König and K. Zeitler, Angew. Chem., Int. Ed., 2011, 50, 951 CrossRef CAS PubMed; (b) T. Keshari, V. K. Yadav, V. P. Srivastava and L. D. S. Yadav, Green Chem., 2014, 16, 3986 RSC; (c) C. W. Kee, K. M. Chan, M. W. Wong and C. H. Tan, Asian J. Org. Chem., 2014, 3, 536 CrossRef CAS; (d) T. Chinzei, K. Miyazawa, Y. Yasu, T. Koike and M. Akita, RSC Adv., 2015, 5, 21297 RSC.
- L. Borkotoky, K. Bora and R. A. Maurya, Asian J. Org. Chem., 2023, 12, e202300146 CrossRef CAS.
- Z. Pan, X. Yang, B. Chen, S. Shi, T. Liu, X. Xiao, L. Shen, L. Lou and Y. Ma, J. Org. Chem., 2022, 87, 3596 CrossRef CAS PubMed.
- S. Tabassum, S. Govindaraju and P. Kendrekar, Top. Catal., 2022 DOI:10.1007/s11244-022-01583-9.
- D. P. Patel, V. K. Patel and S. K. Singh, J. Heterocycl. Chem., 2024, 61, 1455 CrossRef CAS.
- H. K. Singh, A. Kamal, S. Kumari, D. Kumar, S. K. Maury, V. Srivastava and S. Singh, ACS Omega, 2020, 5, 29854 CrossRef CAS PubMed.
- F. Mohamadpour, Front. Chem., 2022, 10, 880257 CrossRef CAS PubMed.
- K. Zheng, K. Liang, J. Zhu, H. Chen, P.-F. Zhang, C. Shen and J. Cao, Mol. Catal., 2024, 565, 114379 CrossRef CAS.
- H.-T. Ji, K.-L. Wang, W.-T. Ouyang, Q.-X. Luo, H.-X. Li and W.-M. He, Green Chem., 2023, 25, 7983 RSC.
- H.-T. Ji, J. Jiang, W.-B. He, Y.-H. Lu, Y.-Y. Liu, X. Li and W.-M. He, J. Org. Chem., 2024, 89, 4113 CrossRef CAS PubMed.
- Q. Shen, X. Zheng, L. Li, T. Zhong, C. Yin and C.-M. Yu, Org. Lett., 2022, 24, 2556 CrossRef CAS PubMed.
- (a) S. Imeni, A. Makarem and R. Javahershenas, Asian J. Org. Chem., 2023, 12, e202300303 CrossRef CAS; (b) M.-K. Yadav and S. Chowdhury, Green Chem., 2023, 25, 10144 RSC; (c) N.-M. Gotgi, J.-S. Jain, R. Pal and D. Ghosh, Org. Biomol. Chem., 2024, 22, 3352 RSC; (d) G.-A. Coppola, S. Pillitteri, E.-V. Van der Eycken, S.-L. You and U.-K. Sharma, Chem. Soc. Rev., 2022, 51, 2313 RSC.
- N. Yang and G. Yuan, J. Org. Chem., 2018, 83, 11963 CrossRef CAS PubMed.
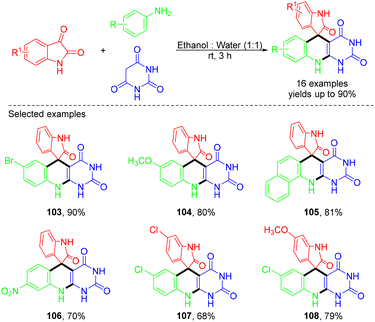
- J.-Y. Chen, H.-X. Li, S.-Y. Mu, H.-Y. Song, Z.-L. Wu, T.-B. Yang, J. Jiang and W.-M. He, Org. Biomol. Chem., 2022, 20, 8501 RSC.
- Y.-H. Lu, S.-Y. Mu, J. Jiang, M.-H. Zhou, C. Wu, H.-T. Ji and W.-M. He, ChemSusChem, 2023, 16, e202300523 CrossRef CAS PubMed.
- Y.-H. Lu, S.-Y. Mu, H.-X. Li, J. Jiang, C. Wu, M.-H. Zhou, W.-T. Ouyang and W.-M. He, Green Chem., 2023, 25, 5539 RSC.
- S. You, M. Ruan, C. Lu, L. Liu, Y. Weng, G. Yang, S. Wang, H. Alhumade, A.-W. Lei and M. Gao, Chem. Sci., 2022, 13, 2310 RSC.
- Y. Kong, Y. Li, M. Huang, J.-K. Kim and Y.-J. Wu, Green Chem., 2019, 21, 4495 RSC.
- Y. Yue, Z. Chen, F. Xue, B. Wang, Y. Zhang, Y. Xia, S. Wu, W. Jin and C. Liu, Org. Chem. Front., 2024, 11, 5813 RSC.
- C. D. J. Cole-Hamilton and R. P. Tooze, Catalyst Separation, Recovery and Recycling, Springer, Netherlands, 2006 Search PubMed.
- B. Baruah and M.-L. Deb, Org. Biomol. Chem., 2021, 19, 1191 RSC.
- N. Vinoth and A. Lalitha, Polycyclic Aromat. Compd., 2020, 42, 517 CrossRef.
- S. Das, S. Paul, B. Mitra, G.-C. Pariyar and P. Ghosh, Results Chem., 2023, 5, 100871 CrossRef CAS.
- X. Li, T. Feng, D. Li, H.-H. Chang, W. Gao and W.-L. Wei, J. Org. Chem., 2020, 85, 9538 CrossRef CAS PubMed.
- Y. Zhang, H. Yan, P. Zhao, R. Chen, W.-W. Fang, L. Wang and Y.-M. Ma, Chem. Commun., 2024, 60, 10712 RSC.
- (a) H. Kiyani and F. Ghorbani, J. Saudi Chem. Soc., 2014, 18, 689 CrossRef; (b) F. Auria-Luna, V. Fernández-Moreira, E. Marqués-López, M. C. Gimeno and R. P. Herrera, Sci. Rep., 2020, 10, 11594 CrossRef CAS PubMed.
- S. Alvi, M. Alam and R. Ali, J. Mol. Liq., 2023, 390, 122951 CrossRef CAS.
- N.-G. Shabalala, N. Kerru, S. Maddila, W.-E. Vanzyl and S.-B. Jonnalagadda, Chem. Data Collect., 2020, 26, 100365 CrossRef CAS.
- V.-S. Bhat and A. Lee, Adv. Synth. Catal., 2022, 364, 3088 CrossRef CAS.
- P. Zhai, W. Li, J. Lin, S. Huang, W. Gao and X. Li, Org. Chem. Front., 2021, 8, 3752 RSC.
- X. Li, P. Zhai, Y. Fang, W. Li, H. Chang and W. Gao, Org. Chem. Front., 2021, 8, 988 RSC.
- (a) R. A. Sheldon, Green Chem., 2005, 7, 267 RSC; (b) A. Farran, C. Cai, M. Sandoval, Y. Xu, J. Liu, M. J. Hernaiz and R. J. Linhardt, Chem. Rev., 2015, 115, 6811 CrossRef CAS PubMed; (c) F. Kerton and R. Marriott, Alternative solvents for green chemistry, Royal Society of Chemistry, 2nd edn, 2013 RSC; (d) B. S. Vachan, M. Karuppasamy, P. Vinoth, S. V. Kumar, S. Perumal, V. Sridharan and J. C. Menendez, Adv. Synth. Catal., 2020, 362, 87 CrossRef CAS.
- For selected reviews on water as a solvent for organic synthesis, see: (a) U. M. Lindstrom, Chem. Rev., 2002, 102, 2751 CrossRef PubMed; (b) M.-O. Simon and C.-J. Li, Chem. Soc. Rev., 2012, 41, 1415 RSC; (c) W. Zhang and B. W. Cue Jr, Organic Synthesis in Water, in Green Techniques for Organic Synthesis and Medicinal Chemistry, ed. M.-O. Simon and C.-J. Li, John Wiley and Sons, 2012 CrossRef; (d) M.-C. Clerget, J. Yu, J.-R. A. Kincaid, P. Walde, F. Gallou and B.-H. Lipshutz, Chem. Sci., 2021, 12, 4237 RSC.
- M. A. E. M. Saleh, J. Conrad, W. Frey and U. Beifuss, Synthesis, 2025, 457 CAS.
- B. Banerjee, A. Priya, M. Kaur, A. Sharma, A. Singh, V.-K. Gupta and V. Jaitak, Catal. Lett., 2023, 153, 3547 CrossRef CAS.
- C. Li, F. Zhang, T. Li, Z. Liu and Z.-L. Shen, Asian J. Org. Chem., 2022, 11, e202200145 CrossRef CAS.
- S. N. Sudheer, S. C. Sahoo, Md. M. Khan and K. Seth, ACS Sustainable Chem. Eng., 2024, 12, 13336 CrossRef.
- H. Wang, Y. Wang, Y. Han, W. Zhao and X. Wang, RSC Adv., 2020, 10, 784 RSC.
| This journal is © The Royal Society of Chemistry 2025 |