Recent advances in electrochemical 1,2-difunctionalization of alkenes: mechanisms and perspectives
Mingming
Zhang†
a,
Ting
Liu†
a,
Xue-Qiu
Chen
a,
Huile
Jin
 a,
Jing-Jing
Lv
a,
Shun
Wang
a,
Jing-Jing
Lv
a,
Shun
Wang
 a,
Xiaochun
Yu
*a,
Chuntian
Yang
*b and
Zheng-Jun
Wang
a,
Xiaochun
Yu
*a,
Chuntian
Yang
*b and
Zheng-Jun
Wang
 *ac
*ac
aInstitute of New Materials and Industrial Technologies, College of Chemistry and Materials Engineering, Wenzhou University, Wenzhou, 325035, P. R. China
bWenzhou Institute of Industry & Science, Wenzhou, 325035, P. R. China
cKey Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), Nankai University, Tianjin 300071, China
First published on 21st January 2025
Abstract
In recent years, significant achievements have been made in the field of electroorganic chemistry regarding the difunctionalization of alkenes. Researchers have developed innovative strategies utilizing the unique reactivity of electrochemical processes to synthesize complex molecules with high regioselectivity and stereoselectivity. This technology is widely applied in the total synthesis of natural products and in the pharmaceutical industry. This article reviews the research progress in the electrochemical difunctionalization of alkenes through three different radical-mediated pathways over the past five years. It includes discussions on 1,2-stereoselective and non-diastereoselective difunctionalization reactions, rearrangements, intramolecular migrations, and cyclization processes. The summary emphasizes innovative electrode designs, reaction mechanisms, and the integration with other emerging technologies, highlighting the potential of this method in modern organic chemistry. Additionally, it aims to address current challenges and propose possible solutions, providing a promising direction for electrochemically mediated difunctionalization reactions of alkenes.
1. Introduction
Over the past decade, the utilization of electrochemical methods to achieve direct functionalization of unsaturated alkenes has garnered significant attention among synthetic chemists. Electrochemical difunctionalization has emerged as an important tool for the synthesis of key natural scaffolds, such as arteannuin B,1 isoocedrene2 and isocryptolepine.3 Compared to traditional organic synthesis methods, electrochemical difunctionalization offers several advantages. Firstly, it eliminates the need for traditional oxidizing or reducing agents. Secondly, reaction conditions are mild. Thirdly, reaction selectivity can be controlled by adjusting the voltage and current. Fourthly, the same electrolytic setup can be used for different types of reactions, facilitating cascade reactions. Lastly, it overcomes some of the challenges encountered in traditional synthesis methods.4–7Despite the significant progress made in recent years, the field of electro-organic synthesis for alkene 1,2-difunctionalizations still faces several challenges. One of the main issues is the relatively low current efficiency and productivity of electrochemical reactions, which limits their practical application on an industrial scale.8,9 This is often due to side reactions, such as electrode passivation, hydrogen evolution, and competitive reactions at the electrode surface. Another challenge is the design and fabrication of efficient electrodes and electrocatalytic cells that can promote electron transfer and enhance the selectivity and yield of the desired products.10 Additionally, the understanding of reaction mechanisms and the development of predictive models for electrochemical reactions are still in their infancy, making it difficult to rationally design and optimize synthetic protocols.
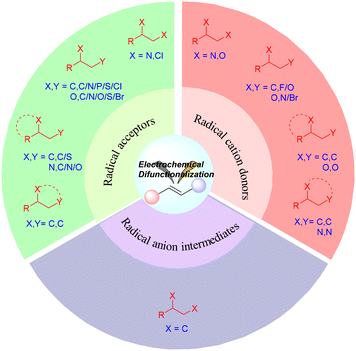
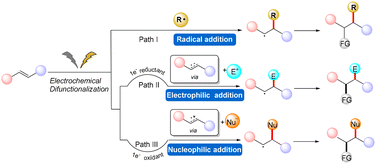
In the context of radical-mediated processes (Fig. 1), the electrochemical difunctionalization of alkenes can be achieved through three main pathways. The first strategy involves the oxidation of a nucleophilic reagent at the anode to generate a radical intermediate. The radical intermediate then undergoes addition to the alkene, forming a carbon-centered radical intermediate. Subsequently, the carbon-centered radical intermediate is oxidized at the anode to generate a carbocation, which finally reacts with the nucleophilic reagent to yield the desired product (as shown in Scheme 1, Pathway I).11–16 The second strategy involves the oxidation of an alkene at the anode to generate a radical cation. The nucleophilic reagent then adds to the radical cation, forming a carbon-centered radical intermediate. The carbon-centered radical intermediate is subsequently oxidized to generate a carbocation, which reacts with the nucleophilic reagent to produce the final product (as shown in Scheme 1, Pathway II).17–28 The third strategy involves the reduction of an alkene at the cathode to generate a radical anion. The electrophilic reagent then adds to the radical anion, forming a carbon-centered radical intermediate. The carbon-centered radical intermediate is further reduced to generate a carbanion, which reacts with the electrophilic reagent to generate the final product (as shown in Scheme 1, Pathway III).29–31
In this review, we aim to examine the research progress over the past five years on electrochemical difunctionalizations of alkenes through three different radical-mediated pathways. This article first delves into relevant electrochemical 1,2-homo/hetero difunctionalization reactions in each section, addressing rearrangements, distal migrations, cyclizations, and dehydrogenative cyclizations. A thorough analysis of their mechanisms follows, highlighting their potential applications and value in organic synthesis. Finally, we explore future prospects and potential development directions in related research fields, aiming to inspire new concepts and advancements in organic synthesis.
2. Alkenes as acceptors to react with diverse additional radicals
Alkenes serving as radical acceptors and their reactions with other diverse radicals represent the most extensively utilized and relatively well-comprehended approaches for achieving double functionalization of unsaturated alkenes. The capacity of these unsaturated compounds to react with free radicals and ion derivatives is ascribed to their π bonds, which are highly reactive loci for various organic reactions. The π bond can function both as a free radical acceptor and, via oxidation, can incorporate various functional groups into organic reactions.32 Furthermore, given the employment of protective/deprotective groups, toxic reagents, complex starting materials, costly transition metals, environmentally detrimental organic solvents, and harsh reaction conditions in conventional double functionalization methods,33 a growing number of scholars have initiated the exploration of the reactions of alkenes as free radical acceptors with different additional free radicals under electrochemical circumstances. This section centers on the dehydrogenative difunctionalization, heterodifunctionalization, rearrangement reactions, and cyclization reactions of alkenes as free radical acceptors and their associated mechanisms.2.1. Dehydrogenative difunctionalization
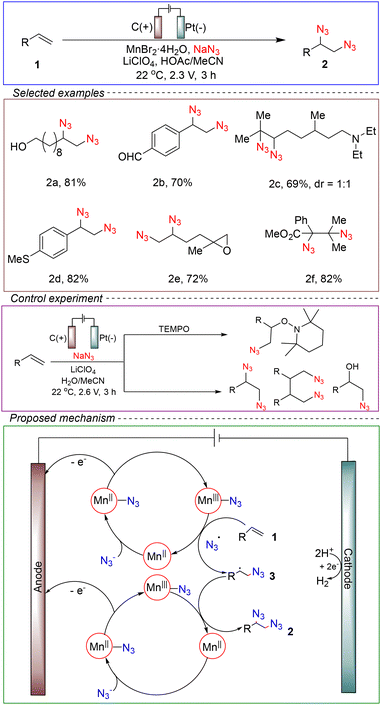
Vicinal diazides are widely used in synthetic organic chemistry as they are crucial precursors of vicinal diamines,34,35 which are important functional motifs found in many biologically active molecules.36 Moreover, vicinal diamines are extensively applied as starting materials for chiral catalysts and ligands used in asymmetric synthesis,37–42 making them an essential component of organic chemistry. The traditional method for obtaining ortho-diamines from alkenes involves the reduction of trimethylsilyl azide and N-fluorobenzenesulfonamide using a Cu(II) catalyst to ultimately synthesize ortho-diamines.43 However, the substrate scope of this method is limited, and it relies on transition metal catalysis. In contrast, electrochemical synthesis, due to its mild reaction conditions and the ability to directly obtain reaction intermediates, presents an ideal alternative method.Lin and co-workers achieved the electrochemical synthesis of ortho-azido diamines for the first time. In a non-divided cell, a carbon felt anode and a platinum cathode were used. Using alkene 1 and sodium azide as model substrates, manganese bromide as the catalyst, and LiClO4 as the electrolyte, a mixed solvent of acetonitrile and acetic acid was employed. The reaction was conducted at a constant voltage of 2.3 V for 3 hours at 22 °C, successfully achieving the electrochemical synthesis of ortho-azido diamines (Scheme 2).44
By conducting control experiments, the important role of the manganese catalyst and a plausible reaction mechanism have been verified. In the absence of a catalyst, the use of a radical scavenger (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO) resulted in 70% yield of the diazo-oxylation product without any diazoamination products. However, in the absence of TEMPO, trace amounts of the diazoamidation product and undesired byproducts were obtained. Therefore, the proposed mechanism suggests that the diazonium ion is first electrochemically oxidized to form a diazo radical. Then, the [MnII]–azide generated from the reaction of sodium azide and [MnII] bromide catalyzes the process. Afterwards, electrocatalysis induced the formation of the [MnIII]–N3 complex. The generated [MnIII]–N3 acted as a potential N3˙ source, which subsequently participated in the addition reactions with alkenes and transferred the diazo group from the [MnIII]–diazenido complex, resulting in the formation of diazooxidation products 2. This mechanism is consistent with the results obtained from voltammetry and spectrophotometry. The advantages of this synthetic method included the use of a manganese catalyst with a low oxidation potential for diazonium ion oxidation, avoiding overoxidation of functional groups.
It also exhibited a wide substrate scope, particularly for primary and secondary amines with oxidation potentials close to those of the active catalyst, which may be attributed to the in situ protection through protonation by HOAc in the reaction medium. Gram-scale reaction experiments could also be performed. The limitation of this method was the high loading of the metal catalyst.
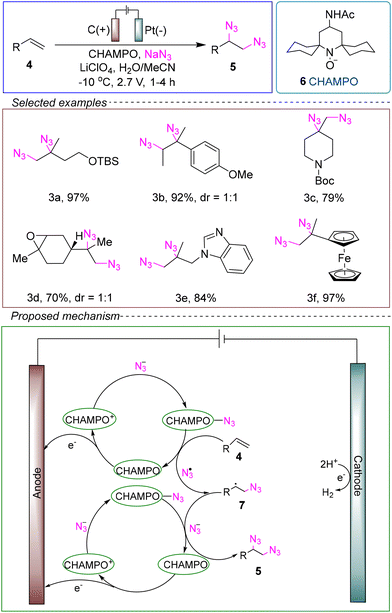
The Kim group developed a novel electrochemical synthesis method that did not require transition metal catalysts. They utilized an undivided cell with a glassy carbon anode and a platinum cathode, employing alkene 4 as the model substrate, NaN3 as the source of diazo radicals, and LiClO4 as the electrolyte. Cyclohexane-based (4-acetamidopiperidin-1-yl)oxy (CHAMPO) 6 served as the catalyst, and a mixed solvent of MeCN and H2O was used. Under conditions of −10 °C and a constant voltage of 2.7 V, the reaction was successfully conducted for 1 to 4 hours to achieve diazotization (Scheme 3).45
Based on experiments with cationic and radical probe substrates, the proposed mechanism suggests that the electrochemical diazotization reaction follows an intramolecular single-electron transfer (SET) cycle and a group transfer cycle. Notably, when the second azide is added to the carbon-centered radical, the CHAMPO–N3 charge transfer complex (CTC) facilitates the transfer of the radical.
In contrast to previous studies that used the radical scavenger TEMPO to produce aminooxy products and promote the oxidation of diazonium ions to diazo radicals, minor amounts of hydrazine derivatives were observed over long reaction times. The innovative use of CHAMPO as a catalyst demonstrated that it can also mediate this reaction while enhancing the yield of the diazo products, likely due to its steric hindrance at the nitrogen–oxygen bond and lower catalyst loading. Another significant feature of this reaction is that it can be performed under neutral pH conditions without transition metals, eliminating the formation of metal diazonium salts and benzoic acid hydrates. Additionally, since NaN3 is soluble in the MeCN and H2O reaction medium, there is no need for an external supporting electrolyte to make the solution conductive. This reaction exhibits broad substrate compatibility and demonstrates promising characteristics in terms of being green, environmentally friendly, and safe. However, this method has some limitations that need to be addressed, including the need for further theoretical data and experimental research to refine the mechanism, as well as the use of high-load of chemical oxidants in the experiments.
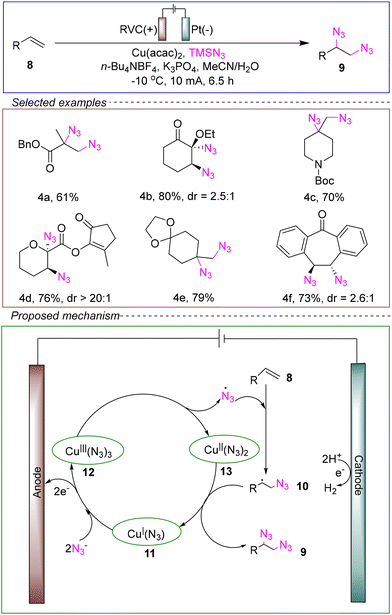
The group of Xu et al. developed a novel copper electrocatalytic diazotization reaction with low catalyst loading. They utilized an undivided cell with a glassy carbon anode and a platinum cathode, employing alkene 8 as the model substrate and TMSN3 as the source of diazo radicals. Tetra-n-butylammonium tetrafluoroborate (n-Bu4NBF4) and potassium phosphate were used as electrolytes, while Cu(acac)2 served as the catalyst. A mixed solvent of MeCN and H2O was employed. Under conditions of −10 °C and a constant current of 10 mA, the reaction was successfully conducted for 6.5 hours to achieve diazotization (Scheme 4).46
The mechanism was proposed through cyclic voltammetry (CV) and control experiments: the anodic oxidation generated [CuIII(N3)3] 12, which released diazo radicals and generated the cuprous azide [CuII(N3)2] 13. The diazido free radicals reacted with alkenes to form alkyls, which then reacted with 13 through ligand transfer to form cupric azide [CuI(N3)] 11. In the presence of N3−, 12 was transferred to the anode to complete the catalytic cycle. The role of K3PO4 is to promote the hydrolysis of TMSN3 to N3−.
The results indicate that the Cu(acac)2 catalyst can effectively and selectively promote the diazotization reactions of different types of alkenes, including some natural products and drug molecules. The metal catalyst used in the reaction was employed in very small amounts. In addition, the system could be used to conduct gram-scale reactions. This method offers advantages such as high efficiency, environmental friendliness, cost-effectiveness, and ease of operation, making it highly promising for industrial applications. Furthermore, the authors observed that the diazotization process occurs at the electrode surface rather than in the bulk solution. This concentration of active copper catalysts and intermediate alkyl radicals in a confined space enhances the local concentration, allowing for a reduction in catalyst loading. This finding provides valuable theoretical support for the design of experiments aimed at lowering catalyst loading in other reactions. However, further theoretical data and experimental investigations were needed to refine the mechanism of this method.
The three mentioned diazotization reactions all involved the generation of N3 radicals from nitrogen sources under specific catalytic conditions. Furthermore, the involvement of radicals in these reactions has been demonstrated through CV and control experiments, providing valuable theoretical insights for future diazotization reactions.
2.2. Heterodifunctionalization
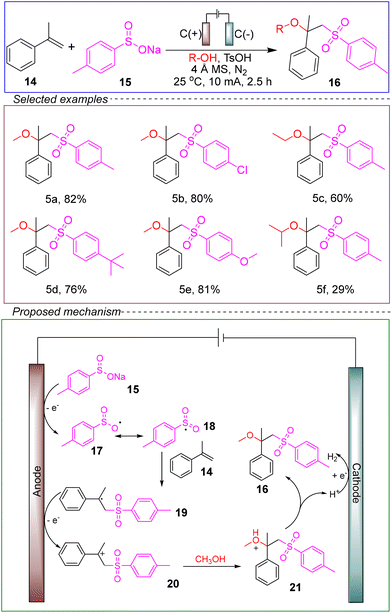
Han's group achieved an electrochemical sulfonation reaction of alkenes. They utilized an undivided cell with a graphite rod anode and cathode, employing α-methylstyrene 14, sodium p-toluenesulfonate 15, and methanol as model substrates. 4 Å MS served as the catalyst, with p-toluenesulfonic acid used as the solvent. Conducted under nitrogen protection at room temperature and a constant current of 10 mA for 2.5 hours, the reaction successfully achieved sulfonation (Scheme 5).58
Using a variable control method, the researchers added the radical inhibitor TEMPO and observed complete inhibition of the reaction, indicating that a radical pathway is involved in the electrochemical transformation. They proposed the following mechanism: sodium p-toluenesulfonate 15 is oxidized at the graphite anode to generate radical 17, which readily undergoes tautomerization to form sulfonyl radical 18. The sulfonyl radical 18 then adds to the C–C double bond of α-methylstyrene 14 to generate the alkyl radical 19. Subsequently, the radical intermediate 19 is anodically oxidized to form the alkyl cation 20, which undergoes a nucleophilic attack with methanol to generate intermediate 21, leading to the target product 16 and the release of H2 at the cathode.
This sulfonation reaction also exhibits good substrate compatibility. However, when sodium p-nitrobenzenesulfinate or aliphatic sulfinate salts are used as substrates, the yields are very low or the reaction does not occur at all. This could be due to the nitro group altering the basicity of the sulfinate, thus affecting the equilibrium in the presence of p-toluenesulfonic acid. This research provides valuable theoretical insights for the future development of electrochemical sulfonation reactions.
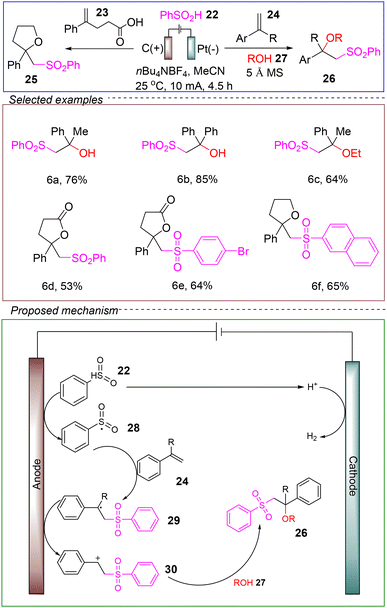
In 2019, Sun and co-workers also utilized sulfinate salts to achieve the electrochemical oxysulfonation of alkenes. They conducted the reaction in an undivided cell with a graphite rod anode and a platinum cathode, using alkene 24 and phenyl sulfinate 22 as model substrates. 5 Å MS served as the catalyst and n-Bu4NBF4 acted as the electrolyte. When a mixture of acetonitrile and water was used, the reaction successfully produced β-hydroxy sulfone after 4.5 hours at 25 °C and a constant current of 10 mA. By substituting water with alcohol 27, the corresponding β-alkoxy sulfone was obtained. This synthetic method is also applicable to 4-phenylpentenoic acid and various aromatic sulfinate substrates, yielding β-carbonyl oxysulfones (Scheme 6).59
The proposed reaction mechanism is as follows: sulfinate 22 is first oxidized at the graphite anode to generate the sulfonyl radical 28. This radical then forms a C–S bond with styrene 24, producing the styrenyl radical 29. The styrenyl radical undergoes an additional oxidation step at the anode to form the styrenyl cation 30. Finally, in the presence of nucleophiles such as water, alcohol, or carboxylic acids, a new C–O bond is formed, resulting in the observed oxysulfone product 26. Concurrently, protons are reduced to hydrogen gas at the cathode, maintaining the current in the closed circuit.
This method demonstrates a broad substrate scope and compatibility with various functional groups, and it does not employ any stoichiometric oxidants during the reaction, highlighting its potential as a green, environmentally friendly, and safe process. However, the method has limitations, including its inability to scale to gram-level reactions and the need for further theoretical and experimental work to refine the mechanism. Overall, this reaction provides a valuable contribution to the development of electrochemical oxysulfonation.
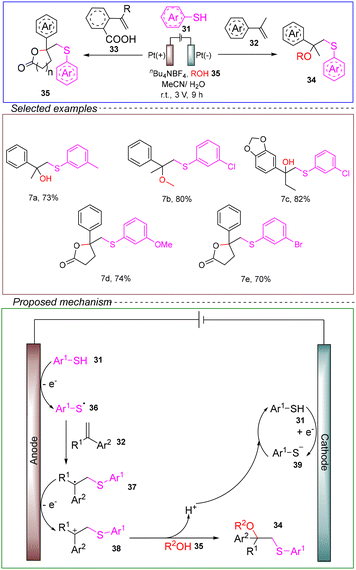
The Mei group achieved the electrochemical oxidative sulfuration of styrene using thiols and nucleophilic water/alcohols. The reaction took place in a non-divided cell with a carbon anode and a platinum cathode, using 1-alkene-2-phenyl 31 and 4-methoxyphenyl ethanol 32 as model substrates, alcohol 35 as the nucleophilic reagent, tetrabutylammonium tetrafluoroborate as the electrolyte, and acetonitrile as the solvent, the reaction was conducted at a constant voltage of 3 V for 9 hours at room temperature, resulting in the electrochemical oxidative sulfuration (Scheme 7).60
The involvement of radical addition in the reaction has been confirmed through CV and control experiments. The proposed mechanism entailed the anodic SET oxidation of thiophene 31, leading to the generation of arylthiyl radicals 36, which subsequently reacted with the corresponding alkene 37. The resulting products underwent oxidation and nucleophilic addition with alcohol/water, yielding the corresponding β-oxysulfuration products 34. Simultaneously, thiophene 31 was reduced at the cathode, liberating hydrogen gas and arylthiolate anions 39 that reacted with hydrogen ions to regenerate thiophene for the next cycle.
This method enabled the dual functionalization of alkenes through cascade oxidative transformations on the anode, without the need for catalysts or oxidants. The reaction exhibited a broad substrate scope. Importantly, the reaction could be scaled up to the gram level with high efficiency. Moreover, this electrochemical system has been successfully applied to the synthesis of sulfur-containing γ-lactones, which are valuable drug molecule frameworks, in high yields. Therefore, this reaction holds promising prospects in terms of being environmentally friendly, sustainable, and suitable for industrial applications. It also provides a valuable and complementary avenue for sulfur compound synthesis.
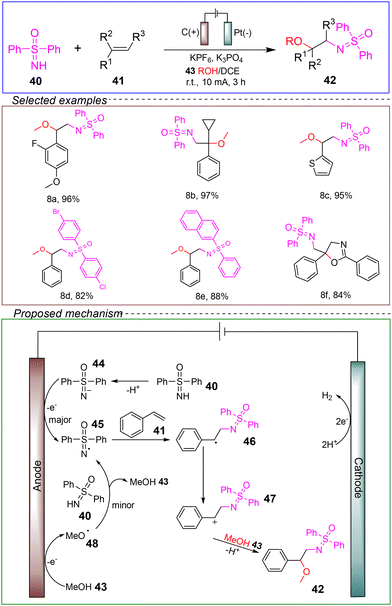
In 2022, Huang's group successfully achieved the electrochemical catalytic oxidation of NH-sulfimines and alcohols with alkenes, synthesizing products containing ortho-sulfimines. They conducted the reaction in an undivided cell with a carbon rod anode and a platinum foil cathode, using NH-sulfimine 40 and styrene 41 as the model substrates. KPF6 and K3PO4 served as supporting electrolytes, and a mixed solvent of methanol 43 and 1,2-dichloroethane (DCE) was employed. The reaction was carried out at room temperature under a constant current of 10 mA for 3 hours (Scheme 8).61
They demonstrated through a radical clock experiment that the reaction involved a benzyl intermediate. Control experiments and HRMS detection indicated the involvement of methoxy radicals and N-centered sulfinyl oxygen intermediates in the electrochemical process. CV experiments confirmed the preferential anodic oxidation of MeOH and NH-sulfilimine. Based on the experimental results, the following mechanism was proposed. The key feature of this mechanism was the formation of N-centered sulfoximidoyl radicals through two pathways: the main pathway involves the oxidation and deprotonation of NH-sulfimine 40 to form the conjugate base 44, which is then oxidized at the anode to generate radical 45. Simultaneously, in the presence of a base, methanol is oxidized at the anode to produce the methoxy radical 48.62,63 The methoxy radical undergoes HAT to form the N-centered sulfonyl oxyl radical 45.64,65 Following this, 45 reacts with styrene to generate the radical intermediate 46, which is further oxidized to form the benzyl cation intermediate 47. The target product 42 is obtained through nucleophilic attack and deprotonation. Additionally, two protons are reduced at the cathode to produce hydrogen gas.
This reaction avoided the need for metal catalysts and external chemical oxidants. Additionally, the method exhibited a broad substrate scope and compatibility with various functional groups. Moreover, the reaction could be scaled up to the gram level with high efficiency. Therefore, the aforementioned reaction holds promising prospects in terms of being environmentally friendly, sustainable, and suitable for industrial applications. It also provides a valuable and complementary avenue for synthesizing organic compounds containing adjacent sulfilimine groups.
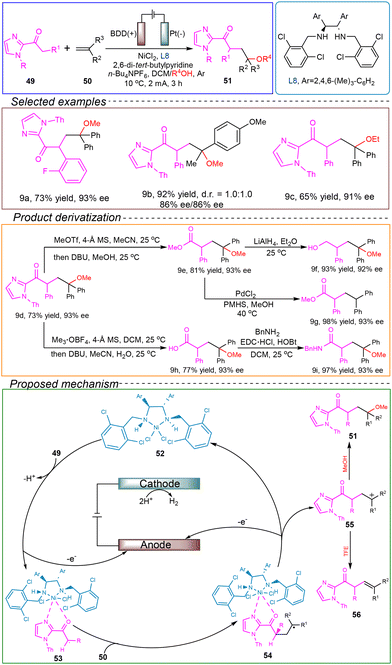
Guo's research group proposed a nickel-catalyzed chiral selective electrochemical coupling reaction between 2-acyl imidazoles and alkenes. This method is a switchable electrocatalytic approach based on anodic oxidation activation, achieving stereoselective organic transformations through the controlled release of chiral α-keto radicals. In an undivided cell with a boron-doped diamond (BDD) anode and a platinum cathode, 2-acyl imidazole 49 and diphenyl ethylene 50 were used as model substrates. 2,6-Di-tert-butylpyridine served as a basic additive, while the chiral diamine ligand L8 acted as the catalyst. n-Bu4NBF4 was used as the electrolyte and p-toluenesulfonic acid was employed as the solvent. The reaction was conducted at 10 °C under a constant current of 10 mA for 3 hours, successfully achieving the coupling reaction (Scheme 9).66
First, they demonstrated through CV that the electrode potential for the nickel-mediated anodic oxidation reaction was significantly lower than the starting potentials required for direct oxidation of different alkenes. Subsequently, using control experiments and a radical clock, they provided evidence for the presence and intermediacy of the α-keto radicals during the conversion process. Based on these findings, the following reaction mechanism was proposed: the reaction began with the addition of the chiral Lewis acid catalyst 52 to the 2-acyl imidazole 49, forming a nickel-coordinated vinyl alcohol acid intermediate. This intermediate underwent oxidation on the anode surface, generating the α-keto radical 53. Radical 53 effectively reacted with electron-rich alkenes, forming the carbon-centered radical 55, which underwent further anodic oxidation to generate the carbon cation intermediate 55 and regenerate the nickel catalyst 52. It is important to note that the nucleophilicity of the alcohol used in the reaction plays a crucial role in determining the final product. When reacting with a nucleophilic alcohol (such as MeOH), the carbon cation 55 reacts to form the bifunctional product 51. In contrast, when a less nucleophilic alcohol (such as TFE) is used, β-H elimination predominates, leading to the formation of the final ethylene product 56.
This method exhibited high efficiency and excellent chemical and enantiomeric selectivity for the synthesis of chiral functionalized products. It also enabled the synthesis of complex imidazole drug molecules, presenting promising prospects for industrial applications. Moreover, this method provided valuable theoretical insights for the development of enantioselective electrochemical transformations.
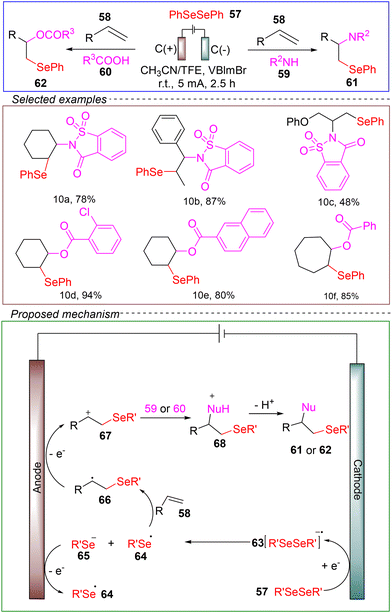
Liu and co-workers reported an electrooxidative method for the amination and oxygen selenylation of alkenes. They utilized an undivided cell with a carbon anode and a carbon cathode, employing activated alkenes 58 as the model substrate, diphenyl diselenide 57 as the selenium source, and 1-butyl-3-vinylimidazolium bromide (VBImBr) as the catalyst. A mixed solvent of acetonitrile and TFE was used. Under conditions of room temperature and a constant current of 10 mA, the reaction was successfully carried out for 2.5 hours to achieve electrooxidative amination and oxygen selenylation (Scheme 10).67
Mechanistic insights into the electrochemical amination and oxygen selenylation process were gained through probe experiments. The carbon radical 66 is generated from the addition of the selenium radical 64 to the carbon–carbon double bond of alkene 58. 66 is then converted to cation 67 through anodic oxidation. Subsequently, the desired product is formed through a sequence of nucleophilic attack and deprotonation.
This reaction is applicable to the sustainable amination and oxygen selenylation of various alkenes, demonstrating a broad substrate scope and compatibility with multiple functional groups. The successful implementation of large-scale experiments and the subsequent modifications of bioactive molecules highlight the potential applications for producing selenium-containing pharmaceuticals. Furthermore, the reaction showcases promising characteristics in terms of being green, environmentally friendly, and safe. Importantly, it also provides a general and modular approach to obtain organic selenocompounds through the electrooxidative difunctionalization of carbon–carbon double bonds from readily available starting materials.
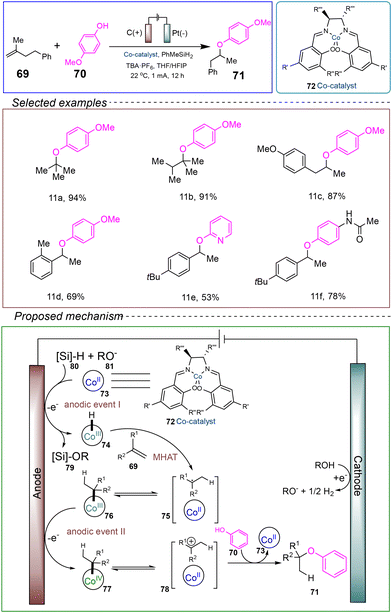
In 2022, Kim's group reported an electrocatalytic hydrogen-etherification reaction involving alkenes and phenols. They utilized an undivided cell with a graphite anode and a platinum cathode, employing alkene 69 and phenolic compound 70 as model substrates. PhMeSiH2 served as the electrolyte, while the Co(II) complex 72 acted as the catalyst precursor. A mixed solvent of tetrahydrofuran (THF) and hexafluoroisopropanol (HFIP) was used. Under conditions of 22 °C and a constant current of 1 mA, the reaction was successfully conducted for 12 hours to achieve hydrogen-etherification (Scheme 11).68
The proposed reaction mechanism is as follows: the Co(II) salen catalyst 73 is anodically oxidized upon the addition of the Si–H bond from hydrosilane 80 to form the Co(III)–hydride 74 (anodic event 1). This Co(III)–hydride 74 undergoes alkene metal hydrometalation (MHAT), concurrently generating the metal/organic radical pair 75. A second anodic oxidation of 75 or the cage-collapse intermediate 76 results in the formation of the corresponding Co(IV) intermediate 77 or a solvent-caged ion pair 78 (anodic event 2). Undesired side reactions, such as HAT or homocoupling, can also occur in conjunction with the escape of radical intermediates from the cage. Finally, the phenolic nucleophile captures the generated intermediates (77 or 78), yielding the desired alkyl aryl ether product 71.
This multifunctional hydrogen-etherification method is applicable to complex molecules, including bioactive compounds, demonstrating a broad substrate scope and compatibility with various functional groups. It also indicates that using nucleophilic phenols as synthetic drug precursors can expand the potential for new drug development. Importantly, this experimental approach successfully employs consecutive oxidative SET reactions, enabling traditional radical-polar cross-coupling reactions to directly capture cationic alkyl intermediates through nucleophilic phenols. Experimental data suggest that using an electrochemical anode as an oxidant is not only sustainable but also offers high reactivity and chemical selectivity, providing a novel reaction mechanism for electrocatalytic hydrogen-etherification. However, this reaction still employs toxic solvents, and further exploration is needed to identify greener, more environmentally friendly, and safer solvents for achieving the electrocatalytic hydrogen-etherification reaction.
An especially interesting part is the trifluoromethyl (CF3) group. The CF3 group significantly affects lipophilicity, permeability, and metabolic stability of compounds, making its effective incorporation into medicinal, agrochemical, and functional organic materials an important area of research in chemistry.77–80 Undoubtedly, new methods that efficiently and selectively incorporate this substituent into different molecular structures have received increasing attention.81–87
However, many trifluoromethylation methods for various functional groups have been extensively described over the past decade.88 Trifluoromethyl sources can be classified according to the electrophilicity or nucleophilicity of CF3 groups, including relatively complex reagents such as Umemoto or Togni reagents or simple and inexpensive chemicals like trifluoromethyl iodide, trifluorochloromethane, triflate salts, or sodium trifluoride. It is worth noting that these methods use expensive CF3 sources,89,90 stoichiometric amounts of oxidants or reductants,88,91 or expensive metal catalysts,90 limiting their applicability in large-scale synthesis.
In recent years, electrochemistry, as a more environmentally friendly alternative, has experienced significant revival in many synthetic routes involving oxidation–reduction processes.92–98 Since electrons are essentially reagents for redox reactions, the use of typically harmful amounts of oxidizing or reducing agents is avoided. Therefore, electrochemical reactions are considered to be safe and “inherently green” methods.99 In this context, the achievement of trifluoromethylation through electrochemical methods has emerged as a powerful and versatile synthetic tool.
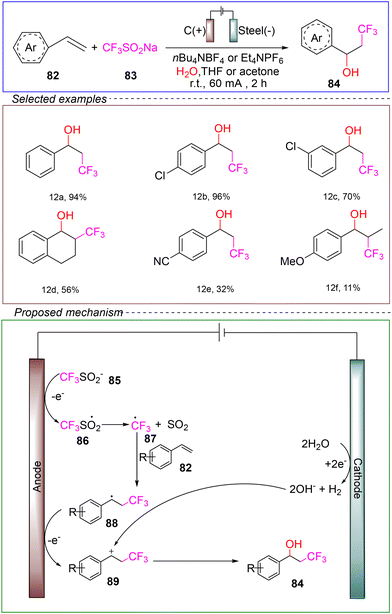
The Kappe group reported an electrochemical trifluoromethylation reaction of styrene. In a non-divided cell, a graphite rod was used as the anode and steel served as the cathode. Using 4-tert-butyl styrene 82 as the model substrate, NaSO2CF3 was employed to provide CF3 radicals, while nBu4NBF4 or Et4NPF6 served as the electrolyte. A mixed solvent of THF or acetone and water was used for the reaction. Conducted at room temperature with a constant current of 60 mA for 2 hours, the reaction successfully achieved trifluoromethylation (Scheme 12).100
By performing CV and radical trapping experiments, it has been demonstrated that trifluoromethanesulfonate underwent anodic oxidation followed by an irreversible reaction. Gas chromatography analysis could detect elusive benzyl radicals. Therefore, based on the aforementioned experiments, the proposed mechanism suggests that the reaction began with the anodic oxidation of the trifluoromethyl anion 85, leading to the formation of the corresponding radical 87. Radical 87 then added onto olefin 82, followed by the oxidation of the olefin to the phenyl cation 89. Finally, the desired product 84 was generated through nucleophilic attack of the hydroxide produced by the cathodic reduction of water.
This method exhibited a broad substrate scope, but it suffered from poor yield when applied to electron-rich alkenes, resulting in excessive formation of by-products. Additionally, the limitation of this reaction method was its inability to achieve gram-scale reactions, and further theoretical investigations were needed to refine the reaction mechanism. Nevertheless, this mild electrochemical approach for the oxygen trifluoromethylation of alkenes undoubtedly established a solid foundation for the development of electrochemical trifluoromethylation methodologies.
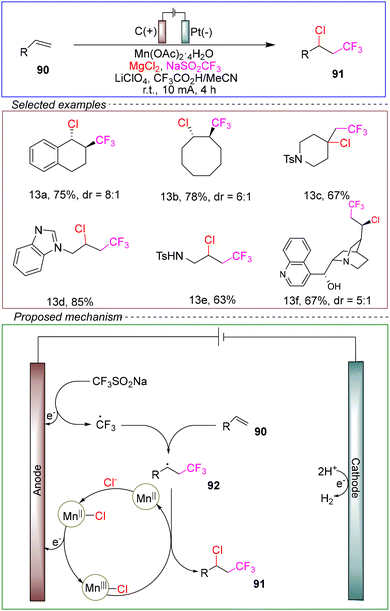
In 2018, Liu and co-workers achieved electrochemical trifluoromethylation. The reaction took place in a non-divided cell with a carbon anode and a platinum cathode. Using 4-tert-butyl styrene as the model substrate, Langlois’ reagent (CF3SO2Na) and MgCl2 provided trifluoromethyl radicals and chloride radicals, respectively. Mn(OAc)2·4H2O served as the catalyst, HOAc acted as the sacrificial oxidant, and LiClO4 was used as the electrolyte, with a mixed solvent of trifluoroacetic acid (TFA) and MeCN. The reaction was conducted at a constant current of 4 mA for 4 hours at room temperature, resulting in electrochemical trifluoromethylation (Scheme 13).101
Control experiments suggested that TFA was not the source of trifluoromethyl radicals but rather enhanced the reactivity of manganese.102,103 The mechanism of this radical trifluoromethylation reaction was similar to that of the manganese-catalyzed diazotization reaction, as shown in Scheme 2.
This method exhibited a wide substrate scope, and the elucidation of its strategy and mechanism would pave the way for innovative approaches in organic systems. It opens new avenues for exploration and innovation in organic synthesis.
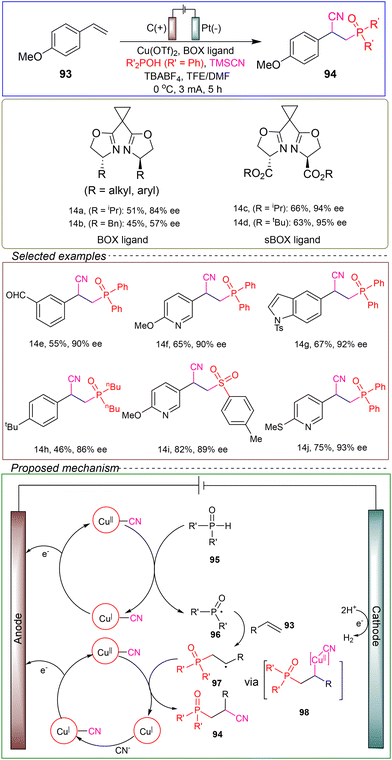
The Liu research group developed a highly enantioselective cyanofunctionalization reaction of styrene using a copper-catalyzed method. In a non-divided cell with a carbon anode and a platinum cathode, they employed 4-methoxystyrene 93 as the model substrate and TMSCN and diphenyl phosphine oxide as reagents. The Cu(BOX) complex was used as the catalyst, with tetra-n-butylammonium tetrafluoroborate (TBABF4) as the electrolyte and a mixture of TFA and MeCN as the solvent. The reaction was conducted at 0 °C under a constant current of 3 mA for 5 hours (Scheme 14).108
Specifically, [CuII]–CN undergoes single-electron oxidation and couples with 95, generating the new P radical intermediate 96. This intermediate then forms a C–P bond with alkene 93, producing a new carbon-centered radical 97. Radical 97 undergoes single-electron oxidation addition to generate the formal [CuII] intermediate 98. Finally, a reduction–elimination step constructs the C–CN bond, resulting in the chiral product 94. CV studies indicate that the oxidative addition step is reversible, while the reductive elimination step is rate-determining and crucial for enantioselectivity. This inner-sphere mechanism enhances the stereoelectronic communication between the transition-state catalyst and substrate, making the reaction suitable for enantioselective catalysis.
To further enhance the enantioselectivity of the reaction, researchers have investigated various chiral ligands commonly used in copper-catalyzed asymmetric catalysis. When employing the bisoxazoline (BOX) ligand, the highest enantioselectivity achieved for the product was 84%. However, when a synthetically derived serine-based ester-substituted BOX ligand (sBOX) was used, the highest enantioselectivity of the product was achieved (95%). Density functional theory (DFT) calculations have shown that the second sphere ester group played a crucial role in inducing high enantioselectivity (vide infra). The experimental method described exhibited a broad substrate scope, and the successful development of selective cyanation reactions further confirmed the robustness of the electrocatalytic strategy as a potential universal platform for the straightforward functionalization of simple alkenes in the vicinity.
This method demonstrates a broad substrate scope and compatibility with various functional groups, and it does not utilize any stoichiometric oxidants during the reaction, highlighting its potential as a green, environmentally friendly, and safe process. However, further theoretical calculations and experiments are needed to clarify the specific reasons for the superiority of the sBOX ligand over the BOX ligand.
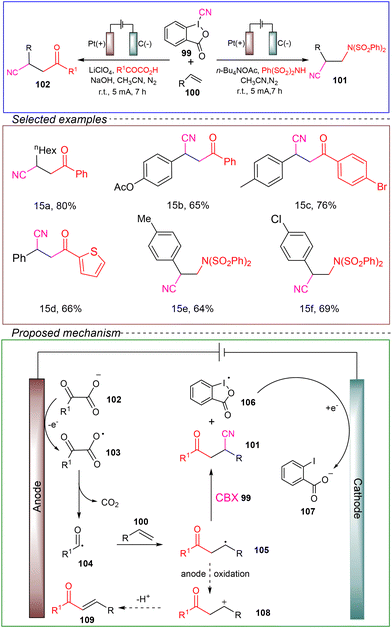
The Kong group investigated a method for the electrocatalytic synthesis of acyl and amino nitriles through a three-component reaction involving simple alkenes. In a non-divided cell with a platinum anode and a carbon cathode, they used 4-bromostyrene 100 as the model substrate, 2-oxo-2-(phenyl)acetic acid as the acyl radical source, and phenyl iodide 99 as the cyano radical source. LiClO4 was employed as the electrolyte, and NaOH served as a basic additive, with acetonitrile as the solvent. Under a nitrogen atmosphere at room temperature and a constant current of 5 mA, the reaction successfully produced acyl nitriles after 7 hours. Following this, the researchers utilized (PhSO2) 2NH as the amination source and n-Bu4NOAc as the electrolyte to synthesize amino nitriles without the addition of a base (Scheme 15).109
Through CV, it could be observed that cyanobenziodoxolone (CBX) 99 was initially oxidized, and the presence of NaOH enhanced the redox capability of CBX 99. Therefore, the mechanism of the acylnitrile synthesis involved the anodic oxidation of α-hydroxycarboxylic acid to generate acyl radical 104 through decarboxylation, which then was added to the alkene to form intermediate 105. Without the use of CBX, intermediate 105 would be further oxidized by the anode, producing carbocation 108, which ultimately underwent deprotonation and yielded the unwanted enone 109 as a side product. However, in the presence of CBX, intermediate 105 could be captured by CBX 99, leading to the formation of the acylnitrile product 101 and intermediate 106. Finally, intermediate 106 was reduced at the cathode to yield the desired acylnitrile 107.
The described method exhibited a broad substrate scope and compatibility with various functional groups. Additionally, the reaction could be conducted at the gram scale with high efficiency. Overall, this reaction holds promising prospects in terms of being environmentally friendly, sustainable, and amenable to industrial applications. However, further theoretical and experimental investigations are required to refine the underlying mechanism. In summary, this method provides a complementary and highly valuable approach for the electrocatalytic synthesis of intermolecular acylnitriles and aminonitriles, filling an important gap in the current repertoire of synthetic methods.
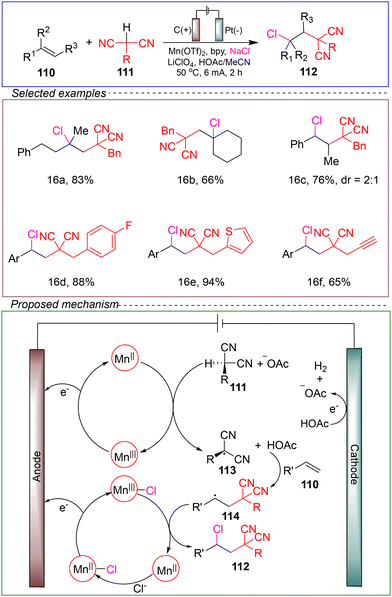
Lin's research group accomplished anodic-coupled electrolysis for the regioselective chloroalkylation of alkenes. They conducted the reaction by using 4-tert-butylstyrene as a model substrate, benzylic acetonitrile as a CN source and NaCl as a Cl source in a non-split cell with a carbon anode and a platinum cathode. Mn(OTf)2 was used as a precatalyst, 2,2-bipyridine (bpy) as a ligand and LiClO4 as an electrolyte. The chloroalkylation reaction was successfully achieved under a constant current of 6 mA at 50 °C in a mixed solvent of HOAc and MeCN for 2 hours (Scheme 16).110
The involvement of carbon-centered radical intermediates in the dual functionalization reaction was further confirmed through atomic clock experiments. Subsequently, a series of control experiments and CV results led to the following proposed mechanism. The mechanism involved the spontaneous generation of both the carbon-centered radical 16 and latent chlorine radical [MnIII–Cl] through the coupled electrolysis process, mediated by Mn catalyst. The new-born transient radical 114 was initially formed through the reaction between the alkene substrate 110 and the carbon-centered radical 113. Then, this transient radical cross-coupled with [MnIII–Cl] (a persistent open-shell intermediate) to produce a bis-functionalized product via a metal-mediated atom transfer-like pathway. Acetic acid generated on the cathode diffused into the bulk solution and served as both a ligand of Mn and a proton acceptor to facilitate the generation of 113.
This method exhibited a broad substrate scope and compatibility with various functional groups. Extensive experimental and theoretical evidence has confirmed the accuracy of its mechanism. This method provides a valuable and complementary perspective for the development of synthetic approaches for electrochemical chloroalkylation reactions.
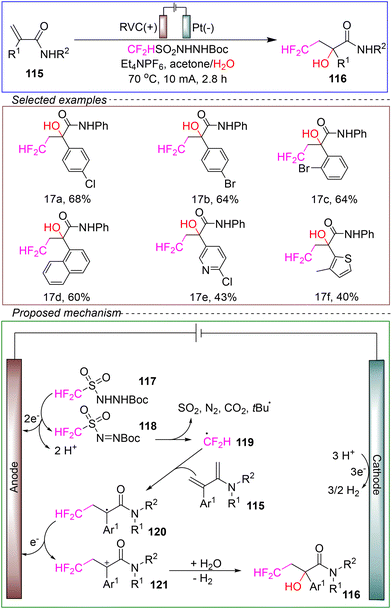
Based on several control experiments, the proposed mechanism is as follows. Reagent 117 underwent anodic oxidation at the anode to generate diazene 118, which subsequently decomposed to form the difluoromethyl radical (˙CF2H) 119. The radical addition of acrylamide 120 to the anode and its one-electron oxidation resulted in the formation of the α-carbonyl carbocation 121. The Ar1 group stabilized the carbon cation, which was crucial for the oxidation of the electron-deficient carbon radical 120. Lastly, carbocation 121 reacted with water to yield the alkene difunctionalization product 116.
This method exhibited a broad substrate scope and compatibility with various functional groups, with moderate yields. However, further experimental and theoretical evidence is needed to confirm the reaction mechanism. Overall, this method serves a valuable complement to the development of synthetic approaches for electrochemical difluoromethylation reactions.
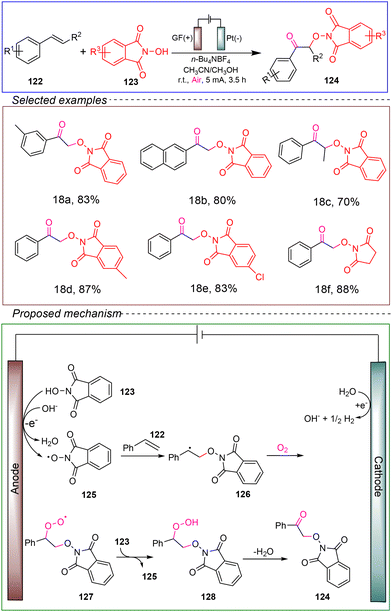
Liu's research group achieved double functionalization of olefins for the preparation of α-oxygenated ketones under electrochemical oxidation conditions. They used styrene 122 and N-hydroxyphthalimide (NHPI) 123 as model substrates, with n-Bu4NBF4 as the electrolyte in an undivided cell with a graphite felt (GF) anode and platinum cathode. The reaction successfully produced α-oxygenated ketones at room temperature under a constant current of 5 mA for 3.5 hours in a mixed solvent of acetonitrile and methanol (Scheme 18).112
The involvement of radical intermediates in the reaction was confirmed through control experiments and isotope labeling. It was demonstrated that oxygen, rather than water, participated in the reaction. CV showed that the N-hydroxyphthalimide 123 was preferentially oxidized in the electrochemical reaction. Based on the above information, a plausible mechanism can be proposed. Initially, the hydroxide anion could be easily reduced from H2O on the cathode. In this reaction, N-hydroxyphthalimide 123 undergoes a proton-coupled oxidation process at the anode, facilitated by hydroxide, resulting in the formation of the N-oxyl radical 125. This radical then adds to styrene, generating the stable carbon radical 126. The carbon radical 126 readily reacts with the oxygen in air, leading to the formation of the peroxo radical 127. Subsequently, the peroxo radical 127 abstracts a hydrogen atom from either N-hydroxyphthalimide 123 or the solvent, producing the corresponding hydroperoxide 128. Finally, hydroperoxide 128 eliminates a molecule of H2O, yielding the target product 124.
This method exhibited a broad substrate scope and compatibility with various functional groups. Furthermore, it allowed for gram-scale reactions and demonstrated high reaction efficiency. Additionally, the reaction employed air as the source of oxygen, eliminating the need for metal catalysts and external corrosive oxidants, and successfully yielded various α-oxygenated ketones. Therefore, based on the aforementioned advantages, this reaction is promising in terms of being environmentally friendly, sustainable, and suitable for industrial application.
2.3. Difunctionalization involving migrations and rearrangements
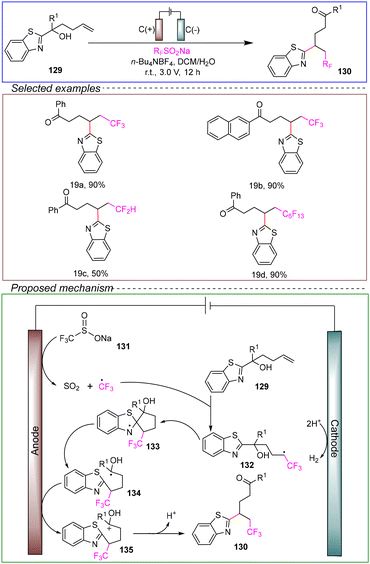
The remote radical migration strategy represents a special type of organic transformation in the field of organic synthesis, with the potential for efficient construction of complex compounds.113,114 Hence, the demand for advanced methods and new reagents is unprecedented.115–124 In particular, intramolecular free radical functional group transfer reactions can be carried out through the formation of cyclic intermediates or transition states, providing an effective strategy for the dual functionalization of C–C double bonds at appropriate positions.The Wang research group successfully achieved a trifluoromethyl/alkenyl/alkynyl-heteroarylation reaction using an electrochemical method. They conducted the reaction in a non-divided cell with a carbon anode and cathode. Using aryl-substituted alkenes 129 as model substrates, CF3SO2Na served as the source of trifluoromethyl radicals. n-Bu4NBF4 was used as the electrolyte, and a mixture of acetone and water acted as the solvent. The reaction was performed at room temperature under a constant voltage of 3 V for 12 hours, successfully achieving the trifluoromethyl/alkenyl/alkynyl-heteroarylation reaction (Scheme 19).125
 | ||
| Scheme 19 Electrochemical trifluoromethyl/alkenyl/alkynyl-heteroarylation of tertiary alcohol tethered with alkene (*acetonitrile is used instead of dichloromethane). | ||
They demonstrated through radical trapping experiments and CV that radical intermediates were involved in the reaction, and NaSO2CF3131 underwent preferential anodic oxidation. Based on the above information, the proposed mechanism could be outlined as follows. Initially, the CF3 radical is generated from 131via anodic oxidation. The addition of the tertiary alcohol 129 leads to the formation of the carbon radical intermediate 132, which rapidly attacks the heteroarene, resulting in the formation of the cyclic nitrogen radical intermediate 133. This cyclic intermediate undergoes fast ring opening followed by radical β-cleavage, yielding the stabilized radical intermediate 134. Intermediate 134 is then oxidized at the anode to form carbocation 135. Finally, deprotonation of carbocation 135 produces the heteroaryl migrated product 130.
They demonstrated through radical trapping experiments and CV that radical intermediates were involved in the reaction, and NaSO2CF3131 underwent preferential anodic oxidation. Based on the above information, the proposed mechanism could be outlined as follows. Initially, the CF3 radical is generated from 131via anodic oxidation. The addition of the tertiary alcohol 129 leads to the formation of the carbon radical intermediate 132, which rapidly attacks the heteroarene, resulting in the formation of the cyclic nitrogen radical intermediate 133. This cyclic intermediate undergoes fast ring opening followed by radical β-cleavage, yielding the stabilized radical intermediate 134. Intermediate 134 is then oxidized at the anode to form carbocation 135. Finally, deprotonation of carbocation 135 produces the heteroaryl migrated product 130.
This reaction involved intricate chemical processes and required specific reaction conditions, including the use of particular solvents, electrolytes, and electrodes, for successful execution. Besides, this method achieved rapid modification of alkenes through cascade dual anodic oxidation without the need for catalysts. It exhibited a broad substrate scope and compatibility with various functional groups, albeit with moderate yields. This radical sequence provides a highly valuable approach for the synthesis of fluorinated ketones and can further elucidate strategies to obtain more functionalized carbonyl compounds.
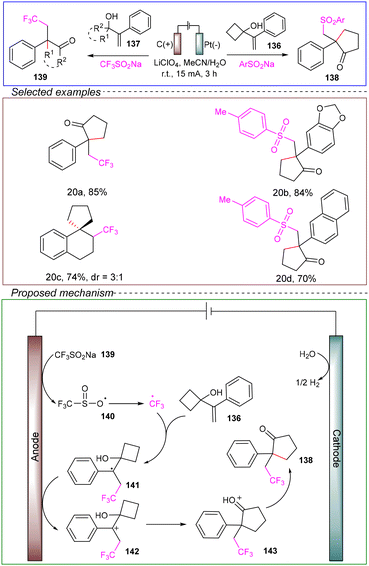
Chen's research group successfully conducted the trifluoromethyl/sulfonyl semi-pyran rearrangement of allyl alcohol using sodium trifluoromethanesulfonate and aryl sulfonate salts. In a non-divided cell with a carbon anode and a platinum cathode, allyl alcohol 136 served as the model substrate, with CF3SO2Na acting as the source of trifluoromethyl radicals. Lithium perchlorate was used as the electrolyte and a mixture of acetonitrile and water served as the solvent. The reaction was performed at room temperature under a constant current of 15 mA for 3 hours, successfully achieving the trifluoromethyl/sulfonyl semi-pyran electrochemical rearrangement (Scheme 20).126
 | ||
| Scheme 20 Electrochemical trifluoromethyl/sulfonylsemipinacol rearrangement reactions of allylic alcohol. | ||
They demonstrated through radical trapping experiments and CV that radical intermediates were involved in the reaction, and the oxidation peak of CF3SO2Na indicated the initiation of CF3 radicals. Based on the above information, the proposed mechanism could be outlined as follows. During the anodic oxidation of CF3SO2Na 139, a SET process generates the sulfonyl radical 140, which then rapidly expels SO2 to form the CF3 radical. Following the addition of the CF3 radical to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, the resulting benzyl radical 141 is further oxidized to form carbocation 142. This intermediate undergoes ring expansion and expulsion to yield the final product 138.
C bond, the resulting benzyl radical 141 is further oxidized to form carbocation 142. This intermediate undergoes ring expansion and expulsion to yield the final product 138.
This method exhibited a broad substrate scope and compatibility with various functional groups, with moderate to excellent yields. Moreover, it avoided the need for stoichiometric oxidants, transition metal catalysts, and harsh reaction conditions. This reaction demonstrates promising prospects in terms of being environmentally friendly, sustainable, and safe. Limitations include the need for further theoretical and experimental investigations to refine the mechanism and the inability to perform gram-scale reactions, which highly impedes its industrial applicability.
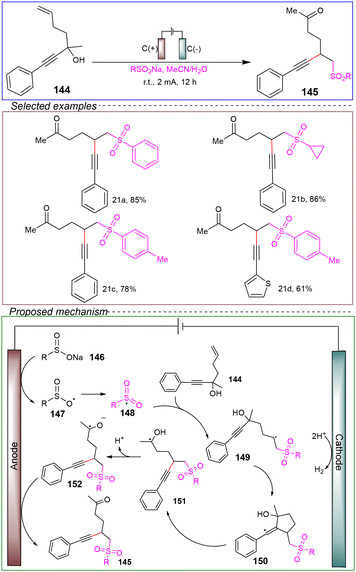
Pan and co-workers successfully applied the constant current electrolysis method to the electrochemical sulfonation of alkynyl-substituted tertiary alcohols tethered to alkenes in an undivided cell. The process was carried out in a solvent mixture of acetonitrile and water (Scheme 21).127
 | ||
| Scheme 21 Electrochemical alkynylsulfonation of alkynyl-substituted tertiary alcohol tethered with olefin. | ||
The involvement of sulfonyl radicals in the reaction was demonstrated through radical trapping experiments. The proposed mechanism was outlined as follows. Initially, sodium sulfinate 146 was anodically oxidized to generate sulfonyl radicals 147, which then reacted with olefins 144 followed by intramolecular cyclization to form vinyl radicals 150. Further cleavage of the C–C bond, dehydrogenation and anodic oxidation led to the final product 145.
This method exhibited a broad substrate scope and compatibility with various functional groups. Additionally, it enabled gram-scale reactions with high efficiency. Furthermore, the reaction did not require any stoichiometric oxidants, additives, or transition metal catalysts, highlighting its potential as an environmentally friendly and sustainable process. A limitation is the need for further theoretical and experimental investigations to refine the mechanism. Overall, this reaction provides a valuable and promising avenue for the development of electrochemical long-range radical migrations.
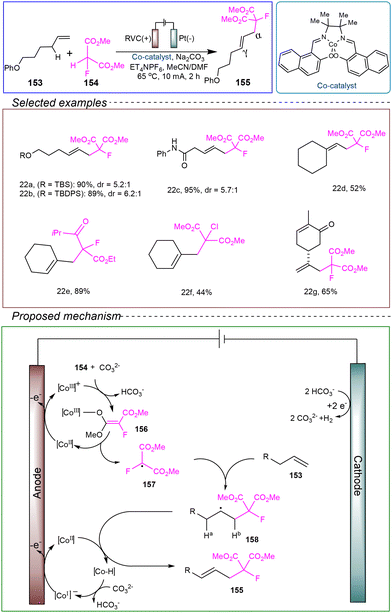
The Xu group reported an electrocatalytic allylic alkylation reaction. They utilized an undivided cell with a RVC anode and a platinum cathode, using α-olefins 153 and 2-fluoropropionic acid esters 154 as model substrates, with Co-catalyst selected for catalysis. Et4NPF6 served as the electrolyte, while Na2CO3 acted as a basic additive, and a mixed solvent of MeCN and DMF was employed. Under conditions of 65 °C and a constant current of 10 mA, the reaction was successfully conducted for 2 hours to achieve the allylic alkylation reaction (Scheme 22).128
The proposed reaction mechanism is as follows: the [CoII] catalyst (Ep/2 = 0.10 V vs. SCE) is oxidized at the anode to generate the [CoIII] complex. With the assistance of a base, this complex oxidizes the acidic carbon nucleophile via intra-ball electron transfer, forming the electrophilic carbon-centered radical 157, which regenerates the [CoII] catalyst. Radical 157 then adds to olefin 153 to generate the new carbon-centered radical 158. The [CoII] catalyst extracts a hydrogen atom (Ha) from radical 158, yielding the final alkylation product 155 and the cobalt species [Co–H]. Computational studies indicate that the HAT of Ha is kinetically more favorable than that of Hb. This kinetic preference can be attributed to steric effects, as Ha is more accessible than Hb for the sterically hindered Co catalyst. The cobalt species [Co–H] is then deprotonated and oxidized back to the [CoII] catalyst at the anode, while protons are reduced to H2 at the cathode.
This reaction operates under mild conditions, and the cobalt catalyst demonstrates a low oxidation potential and high efficiency, ensuring excellent tolerance toward various functional groups. However, the use of toxic solvents remains a concern, and further investigation is needed to explore greener, more environmentally friendly, and safer solvents for achieving the allylic alkylation reaction.
2.4. Cyclization
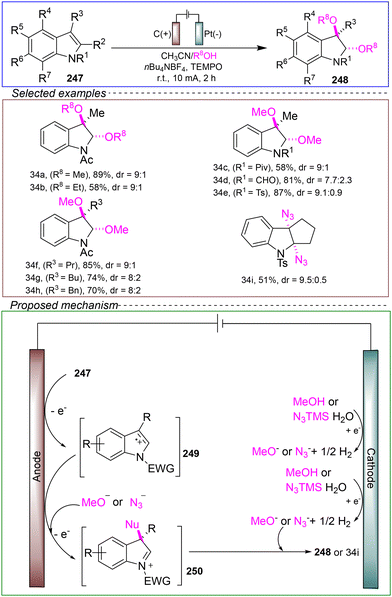
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 132–134 groups can significantly improve their physical, biological, and chemical properties, leading to significant interest in trifluoromethylated morpholines.
132–134 groups can significantly improve their physical, biological, and chemical properties, leading to significant interest in trifluoromethylated morpholines.
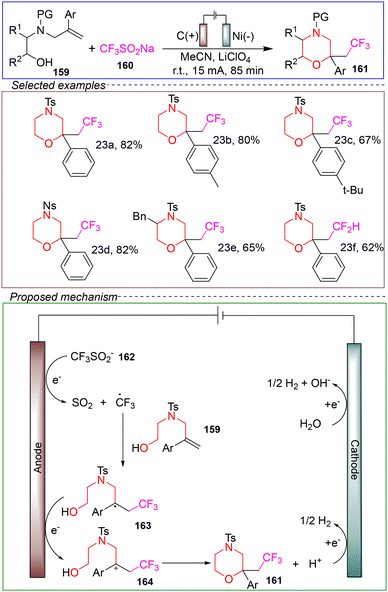
In 2020, Masson's group developed an electrochemical-mediated trifluoromethylation method for synthesizing enol trifluoromethyl-substituted pyrrole derivatives. This method employs mild reaction conditions and utilizes inexpensive and easy to handle sodium trifluoromethanesulfonate as the trifluoromethylation reagent. In an acetonitrile solution, the reaction was conducted with carbon as the anode and nickel as the cathode under a constant current of 15 mA at room temperature for 85 minutes, successfully yielding a variety of substituted pyrrole derivatives (Scheme 23).135
 | ||
| Scheme 23 Electrochemically mediated enol trifluoromethylation to synthesize morpholine derivatives. | ||
Based on control experiments and CV, the following mechanism was proposed. The SET anodic oxidation of CF3SO2Na 160 led to the release of SO2 and the generation of CF3 radicals. Subsequently, the electrophilic CF3 radicals were selectively added to alkene 159, generating the radical intermediate 163. This intermediate underwent a second SET anodic oxidation, further oxidizing it to cation 164. Finally, the intramolecular nucleophilic addition of the alcohol part led to the formation of the corresponding trifluoromethylated pyrrole 161, accompanied by the release of H+. The reduction of the hydrogen ions and residual trace amounts of water occurred at the cathode.
This method exhibits a broad substrate scope and compatibility with various functional groups, and it does not utilize any stoichiometric oxidants during the reaction, highlighting its potential as a green, environmentally friendly, and safe process. However, there are some limitations. Firstly, the reaction cannot be scaled to gram-level amounts. Secondly, the method is only applicable to the transformation of arenes; when using alkyl alkenes as substrates, the corresponding morpholine products are not obtained.
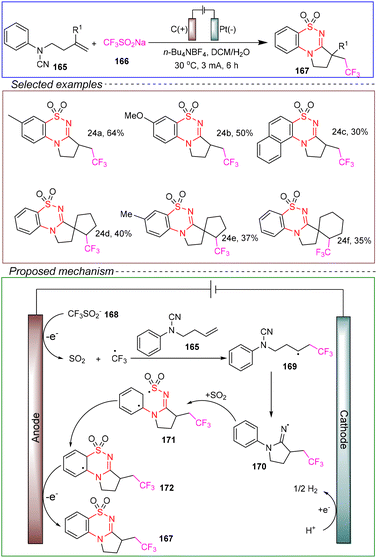
Liao's group achieved an electrochemical tandem trifluoromethylation cyclization reaction. They conducted the reaction in a non-divided cell with a carbon anode and a platinum cathode, using N-(but-3-en-1-yl)-N-phenylcyanamide 165 as the model substrate and CF3SO2Na 166 as the source of the trifluoromethyl radical. n-Bu4NBF4 was used as the electrolyte, and a mixture of DCM and water served as the solvent. The reaction was performed at 30 °C under a constant current of 3 mA for 6 hours, successfully achieving trifluoromethylation cyclization (Scheme 24).136
Based on control experiments, the mechanism was as follows. The anodic oxidation of the trifluoromethanesulfonate anion 168 generates the corresponding radical, which decomposes via desulfurization to form the CF3 radical and SO2. The CF3 radical then attacks the alkene part of cyanamide 165, resulting in the formation of the c-radical intermediate 169. This intermediate undergoes intramolecular addition to the cyano group, yielding the imino radical intermediate 170. The imino radical 170 captures SO2, transferring the sulfonyl radical 171, which further cyclizes to form intermediate 172. Finally, further anodic oxidation and aromatization yielded the corresponding product 167.
This method showcases a broad substrate scope and compatibility with various functional groups, and it can be scaled to gram-level reactions. Importantly, the reaction does not employ any stoichiometric oxidants, underscoring its potential as a green, environmentally friendly, and safe process. Additionally, it provides theoretical support for the design of other sulfonation reactions in the future.
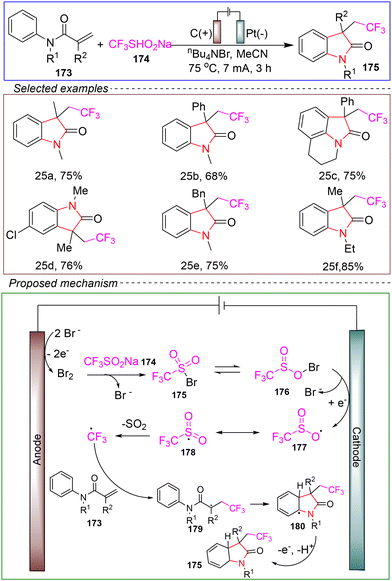
Zeng's group developed an electrochemical trifluoromethylation method for N-arylacrylamides catalyzed by bromine. The reaction was carried out in an undivided cell with graphite as the anode and platinum wire as the cathode, using tetrabutylammonium bromide as the electrolyte. The reaction was performed at 75 °C under a constant current of 7 mA for 3 hours, and trifluoromethyl radicals were generated indirectly via electrolysis of sodium trifluoromethanesulfinate to convert N-arylacrylamides into trifluoromethylated indolinones through cyclization (Scheme 25).137
Based on these control experiments and CV tests, the following reaction mechanism was proposed. Molecular Br2 is initially generated by the anodic oxidation of bromine. This is followed by a reaction with sodium CF3SO2Na 174 to form sulfonyl hypobromite 176, which reaches an equilibrium with intermediate 175. The generated intermediate 176 or 175 then undergoes cathodic reduction to form the oxygen-centered radical 177 or the sulfur-centered radical 178, respectively, while bromide ions are regenerated. Subsequently, the rapid loss of an SO2 molecule produces the CF3 radical. Once the CF3 radical is formed, it combines with acrylamide 173 to generate radical 179. Finally, intramolecular cyclization and further aromatization occur under anodic oxidation conditions, yielding the corresponding product 175.
This reaction demonstrates a broad substrate scope and compatibility with various functional groups, although the yields are generally moderate. The use of low-load catalysts highlights its cost-effectiveness and scalability, suggesting that this reaction has promising features such as being green, environmentally friendly, and safe. It provides a new feasible method for effectively achieving trifluoromethyl cyclization.
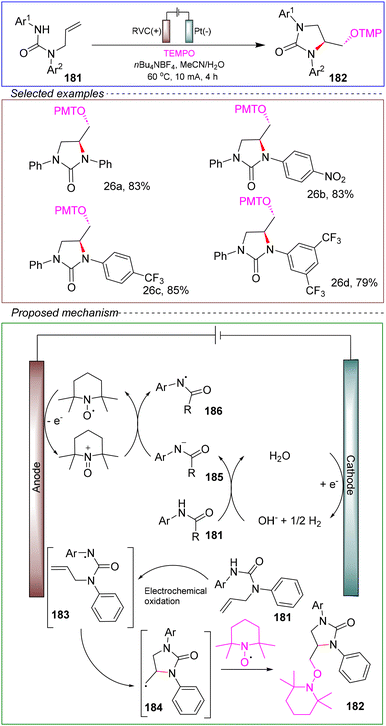
Based on these control experiments and cyclic voltammetry tests, the following reaction mechanism has been proposed: using urea as a substrate 181. The process begins with the anodic oxidation of TEMPO, generating ammonium ions, while the cathodic reduction of H2O produces OH− and H2. Substrate 181 is then deprotonated by the electro-generated hydroxide to form a nitrogen-containing anion 185, which acts as a better electron donor than the neutral precursor. SET occurs between the nitrogen-containing anion 185 substrate and the ammonium ion, providing a radical with a deficit of electrons at the nitrogen center and regenerating the TEMPO radical. Subsequently, the nitrogen radical intermediate can cyclize onto the urea ring, generating another radical at the terminal carbon, which reacts with the TEMPO radical molecule to form the bifunctional product 182.
This reaction constitutes a general and mild method for the difunctionalization of alkenes and the construction of cyclic ureas with high yields by adding nitrogen radicals to unactivated/unsubstituted terminal alkenes in the presence of TEMPO. In this process, TEMPO serves a dual role as a redox promoter and a radical trap. The reaction also demonstrates a broad substrate scope and compatibility with various functional groups. This approach is characterized by its low cost, lack of oxidants and catalytic conditions, and good scalability, suggesting that the reaction has promising features such as being green, environmentally friendly, and safe. It provides a feasible method for the electrochemical amination reaction to synthesize cyclic ureas.
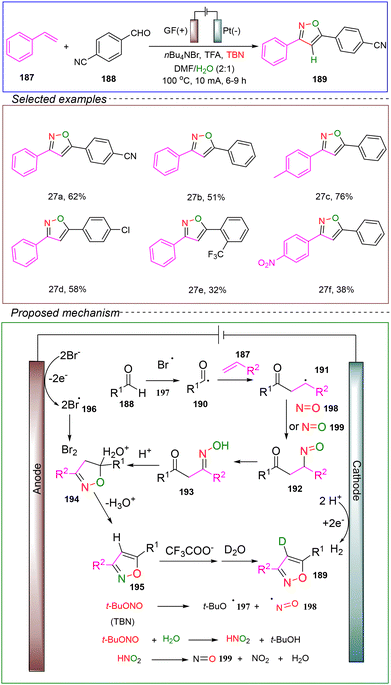
The Ackermann group reported a four-component domino reaction. They utilized an undivided cell with a graphite felt anode and a platinum plate cathode, employing 4-cyanobenzaldehyde 188 and styrene 187 as model substrates, with TBN serving as both substrate and oxidant. The electrolyte used was n-Bu4NBr, and a mixed solvent of DMF, TFA, and water was employed. Under conditions of 100 °C and a constant current of 10 mA, the reaction was successfully conducted for 6 to 9 hours to achieve the electrooxidative [1 + 2 + 1 + 1] cycloaddition (Scheme 27).139
A detailed mechanism was proposed through isotopic labeling, kinetic studies, CV analysis, and investigations into the reactivity of intermediates. Initially, bromide ions from the electrolyte are oxidized at the anode to generate the bromine radical 196. Concurrently, under thermal conditions, TBN undergoes homolysis to produce radicals 197 and 198. The reaction began with the formation of the acyl radical 190, which results from the in situ reaction of aldehyde 189 with radical 196 or 197. Subsequently, radical 190 attacks the double bond of the alkene, generating the carbon-centered radical 191. With the assistance of nitroso radicals 198 or 199, compound 192 is formed. This is followed by an isomerization process, rapidly leading to the key intermediate β-carbonyl ketoxime 193. The addition of TFA promotes the dehydration of intermediate 193, facilitating the formation of the required isoxazole 195. Under the influence of carboxylate ions, 195 may experience hydrogen loss at the 4-position of the isoxazole framework. It can then extract a proton from a deuterated water molecule, leading to the formation of the deuterated fragment 189.
This study not only provides detailed experimental evidence supporting the proposed mechanism but also demonstrates the significant potential for creating various heterocyclic compounds, showcasing the broad substrate scope and compatibility with multiple functional groups. In summary, this reaction offers an innovative electrochemical cyclization strategy, with promising applications in drug discovery and materials science.
In 2020, Lei's group reported an electrochemical intermolecular dehydrogenative [4 + 2] cyclization reaction between indole-1H-carboxamide and 1-alkylindole to synthesize hexacyclic indoles. They employed an undivided cell with a platinum anode and cathode, using 1,3-dimethylindole 200 and N,5-dimethoxyindole-1H-carboxamide 201 as model substrates. Et4NBF6 served as the electrolyte, while PhCOONa acted as a basic additive, and a mixed solvent of HFIP and DMF was utilized. Under nitrogen protection and a constant current of 5 mA at room temperature, the reaction was successfully conducted for 160 minutes to achieve [4 + 2] cyclization (Scheme 28).140
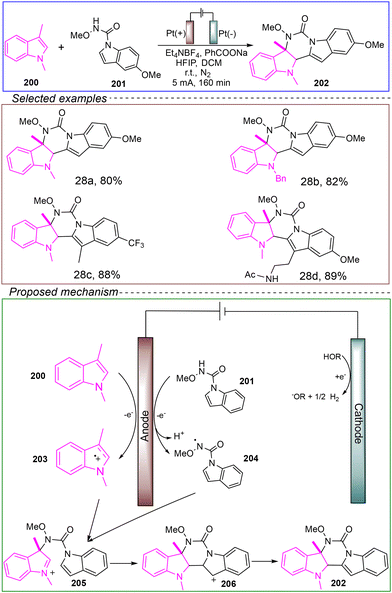 | ||
| Scheme 28 Electrooxidation enables selective dehydrogenative [4 + 2] annulation between indole derivatives. | ||
The proposed reaction mechanism was elucidated through electron paramagnetic resonance (EPR), radical trapping, and CV experiments. Initially, 200 is oxidized at the anode to generate the indole radical cation 203. Meanwhile, under the assistance of an equivalent base, 201 undergoes SET oxidation to produce the N-centered radical 204. The indole radical cation 203 then undergoes a radical–radical cross-coupling with the N-centered radical 204, forming intermediate 205. Following intramolecular cyclization and deprotonation, the highly regioselective pyrido indole 202 is produced. The cathodic reduction of HFIP releases hydrogen gas during the reaction.
This reaction demonstrates a broad substrate scope and compatibility with various functional groups, making it a valuable strategy for drug research and other functionalization applications. Furthermore, mechanistic studies propose a new pathway in which the electrooxidative cyclization occurs via a radical–radical cross-linking between the indole radical cation and the n-centered radical. However, the reaction still employs toxic solvents, and further investigation is needed to explore greener, more environmentally friendly, and safer solvents for achieving the electrochemical intermolecular dehydrogenative [4 + 2] cyclization reaction.
Tang's group achieved the electrochemical synthesis of 1-naphthol through intermolecular cyclization of alkenes with 1,3-dicarbonyl compounds. They conducted the reaction in a non-divided cell with a carbon anode and a platinum cathode, using ethyl benzoylacetate 207 and phenylacetylene 208 as the model substrates. Sodium ethoxide (NaOEt) served as a basic additive, while ferrocene (Cp2Fe) was utilized as the catalyst. Tetraethylammonium thiosulfate (Et4NOTS) was employed as the electrolyte, and a mixture of THF and ethanol acted as the solvent. The reaction was performed at 100 °C under a constant voltage of 1.15 V for 2 hours, successfully achieving the (4 + 2) cyclization to synthesize 1-naphthol (Scheme 29).141
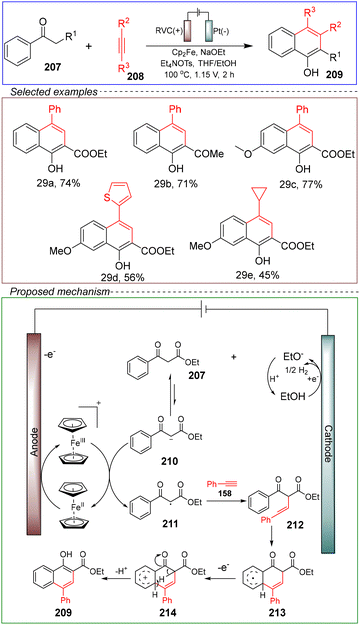 | ||
| Scheme 29 Electrochemical oxidative annulation of 1,3-dicarbonyl compounds with alkynes catalyzed by ferrocene. | ||
Through variable control and CV experiments, the mechanism proposed was as follows. Under electrochemical conditions, ethanol undergoes a reduction reaction at the cathode, generating an ethoxy anion and H2. The ethoxy anion reacts with the 1,3-dicarbonyl compound 207 to form the carbon intermediate 210. The ethoxy anion is derived from the reduction of ethanol and the addition of NaOEt. Meanwhile, at the anode, Cp2Fe is oxidized to Cp2Fe+, which is further oxidized to intermediate 210, generating the C-radical intermediate 211. This radical intermediate 161 then reacts with acetylene 208 to form intermediate 212, which undergoes intermolecular cyclization to yield intermediate 213. Finally, oxidation leads to intermediate 214, which undergoes deprotonation to produce 1-naphthol 209. The released protons can combine with the ethoxy anion to complete the reaction cycle.
This method for synthesizing naphthol derivatives through intermolecular (4 + 2) cyclization demonstrates the use of inexpensive ferrocene as a redox catalyst, allowing for the release of H2 and eliminating the need for precious metal catalysts and external oxidants. The process is environmentally friendly, safe, and sustainable. Importantly, it also enables the synthesis of derivatives with significant anticancer activity, providing valuable resources for in vitro cytotoxicity screening and related medical applications.
3. Alkenes as radical cation donors
Compared to the commonly used radical-mediated functionalization of alkenes, where they act as radical acceptors, the alkene-derived radical cationic strategy presents a distinct transformation pathway that expands the coupling partners available to alkenes.142,143 Difunctionalization of alkenes with two nucleophiles is conventionally challenging. However, the single electron oxidation (SEO) strategy provides an opportunity to convert the alkene to a more electrophilic radical cation, which was first captured by a nucleophile and then converted into a carbocation intermediate by SEO. Finally, the intermediate underwent functionalization using another nucleophile. Because alkenes are inherently oxidizable, they typically possess higher oxidation potentials. This observation suggests that generating reactive alkene-derived radical cation species via SEO requires a potent oxidation system.Electrocatalysis has been established as an effective and environmentally sustainable approach for the synthesis of alkenes, and it offers a technically appealing platform for SEO.144–154 It is noteworthy that these strategies fundamentally change the transformation of alkenes. They initiate the formation of radical cation intermediates derived from alkenes, which can then be captured by nucleophiles or unsaturated hydrocarbons. This new approach is significantly different from the previously widely studied electrophilic radical mechanisms, providing new perspectives and possibilities for alkene reactions. It is worth noting that one of the main challenges associated with electrochemical reaction systems is that a high reaction potential can lead to the loss of an electron from the alkene, which often results in unwanted overoxidation.155,156 In some electrochemical reactions that involve redox media with reduced oxidation potentials, preventing overoxidation is usually necessary.157–161
We provide an overview of the latest developments in the utilization of electro-alkene-derived radical cations in synthetic chemistry, which includes difunctionalization, cyclization, and other transformation types. Special attention has been given to the SEO mechanism of alkenes, with the purpose of encouraging synthetic chemists to create new and effective catalytic systems. Furthermore, this section also covers the substrate scope, as well as potential applications and limitations associated with these transformations.
3.1. Dehydrogenative difunctionalization
The radical-mediated difunctionalization of alkenes, which involves the incorporation of two functional groups, is a highly efficient and streamlined synthetic technology that has been widely applied over the past few decades in various fields such as synthetic chemistry, medicinal chemistry, and materials science for constructing valuable organic skeletons.162–164 However, in many well-established difunctionalization reactions of alkenes, additional radical species are needed to attack the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond and generate the C(sp3) radical species. Using two nucleophiles to achieve difunctionalization of alkenes is a challenging task. However, the optimization of electrochemical catalysis offers a way to overcome this limitation. This section will focus on reviewing the applications of alkene-derived radical cation chemistry in the synthesis of bifunctionalized alkenes.
C bond and generate the C(sp3) radical species. Using two nucleophiles to achieve difunctionalization of alkenes is a challenging task. However, the optimization of electrochemical catalysis offers a way to overcome this limitation. This section will focus on reviewing the applications of alkene-derived radical cation chemistry in the synthesis of bifunctionalized alkenes.
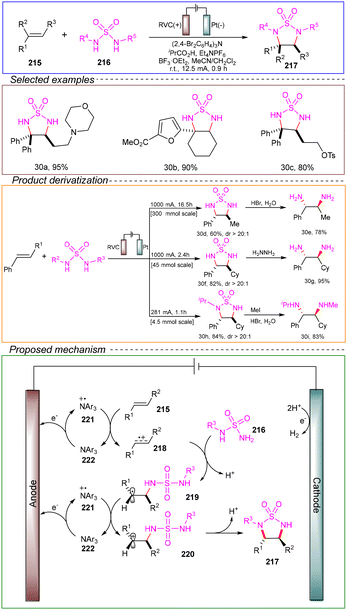
Through variable control and CV experiments, the proposed reaction mechanism was as follows. Substrate 219 undergoes single-electron transfer oxidation by oxidant 221, generating carbocation 220. This carbocation can then cyclize to form the cyclic sulfonamide 217. The cyclization of 220 plays a crucial role in controlling the stereoselectivity of the 1,2-diamination, as the substituents R1 and R2 on the alkene are situated on opposite sides of the newly formed five-membered ring, reducing steric hindrance. Electrons lost from the alkene to the anode ultimately combine with protons at the cathode to form H2, eliminating the need for external electron and proton acceptors.
This reaction reports a highly novel electrochemical 1,2-diamination method, demonstrating a broad substrate scope and compatibility with various functional groups. It can also be scaled to gram-level reactions, and notably, it does not utilize any stoichiometric oxidants during the process, underscoring its potential as a green, environmentally friendly, and safe approach. The comprehensive mechanistic investigation provides substantial theoretical and technical guidance for further development of electrochemical diamination reactions.
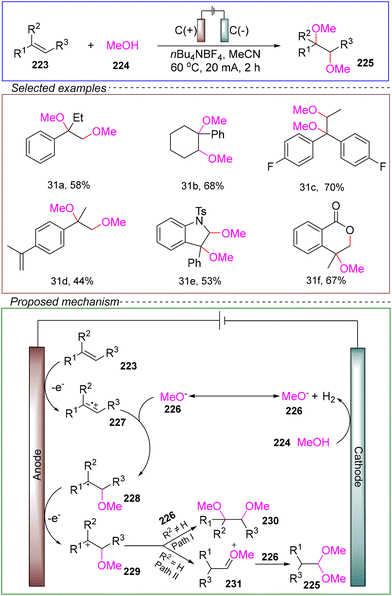
Xu's group developed an electrochemical dimethoxylation reaction of alkenes. In a non-divided cell with platinum as both the anode and cathode, they used 1,1-diphenylethylene 223 and methanol 224 as model substrates, with n-Bu4NBF4 as the electrolyte and acetonitrile as the solvent. The reaction was conducted at 60 °C under a constant current of 20 mA for 2 hours, successfully achieving dimethoxylation (Scheme 31).174
Based on control experiments and CV, the proposed reaction mechanism was as follows. First, anodic oxidation of the alkene generates the radical cation intermediate 227, which subsequently undergoes nucleophilic addition to form radical 228. Further oxidation of the transient species 228 leads to the formation of carbocation 229. Due to the rapid steps of nucleophilic addition and radical oxidation, carbocation 229 serves as the main intermediate. Depending on the stability of 229, two different reaction pathways may occur. The more stable carbocation, containing multiple substituents, can undergo direct nucleophilic addition (Path I), while the less stable carbocation can produce acetal product 225 through a semi-naphthol rearrangement (Path II).
The results indicate that this dimethoxylation reaction is compatible with various alkenes, demonstrating a broad substrate scope. Additionally, it can be scaled to gram-level reactions, highlighting its potential as a green, environmentally friendly, and safe method. Importantly, this approach provides a complementary method to traditional alkene reactions with various added radicals, offering a theoretical foundation for future studies exploring alkenes as radical cation donors in dimethoxylation reactions.
Despite significant progress in the field of olefin epoxidation, there still exist issues with the stereoselective preparation of 1,2-diol derivatives. Kim's research group successfully synthesized syn-1,2-diol derivatives through electrochemical methods. In a non-divided cell with a carbon anode and a platinum cathode, they used 4-tert-butylstyrene 232 as the model substrate, TBABF4 as the electrolyte, and trifluoroacetic acid and water as additives, with DMF as the solvent. The reaction was conducted at 22 °C under a constant voltage of 2.5 V for 12 hours, successfully yielding the protected 1,2-diol (Scheme 32).175
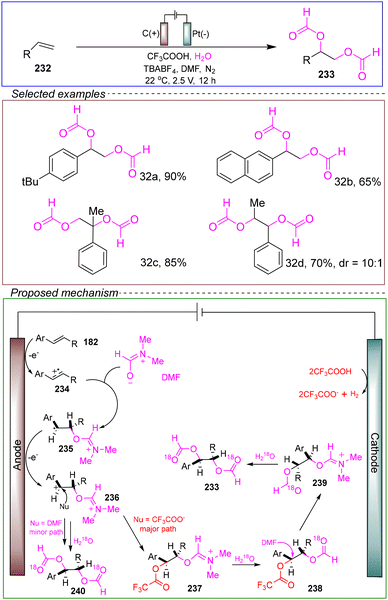 | ||
| Scheme 32 Electrochemical functionalization of alkenes to construct protected syn-1,2-diol derivatives. | ||
This method provided a practical and convenient strategy for the stereoselective dimethoxylation of protected syn-1,2-diol derivatives since the methoxyl groups could be easily converted to hydroxyl groups. The proposed mechanism first involved the oxidation of olefins to radical cations at an appropriate reaction potential. The radical cation was then captured by DMF to form a carbon-centered radical, which further was oxidized to a cation at the anode surface. On the one hand, intermediate 235 reacted with DMF as the nucleophile in the presence of water to yield an anti-dimethoxylation product with a leaving group of HNMe2. It is noteworthy that this pathway is not considered as a major process due to the production of by-products. On the other hand, intermediate 236 was trapped by a better nucleophile, trifluoroacetic acid, to form intermediate 237. Finally, the target stereoselective dimethoxylation product was formed via nucleophilic substitution and hydrolysis processes. Undoubtedly, the observed highly non-enantioselective product could be attributed to the formation of intermediate 237, which occurred before the non-enantioselective control erosion caused by the rotation of the C–C bond.
The results indicate that this reaction demonstrates a broad substrate scope and compatibility with various functional groups, and it can be scaled to gram-level reactions, highlighting its potential as a green, environmentally friendly, and safe method. Furthermore, the study introduced a simple method for the deprotection of the formyl protecting group from the diol product. This provides a solid theoretical foundation for further developing nucleophilic alkene functionalization reactions using trifluoroacetic acid in future studies.
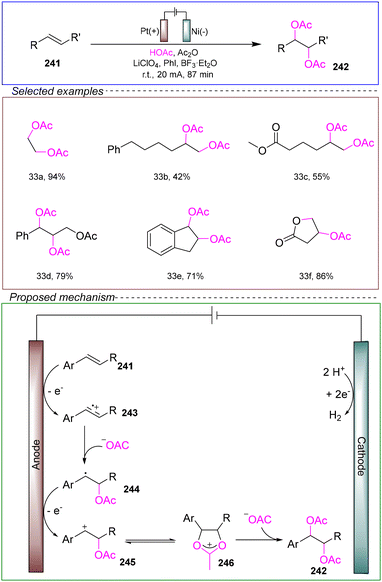
In 2023, De Vos and co-workers reported a highly effective electrocatalytic diacetoxylation method. They utilized an undivided cell with a platinum anode and a nickel cathode, using 1-octene 241 as the model substrate, PhI as the catalyst, and a mixture of BF3·Et2O and LiClO4 as the electrolyte. A solvent mixture of acetic acid and acetic anhydride was employed. Under these conditions, the reaction was carried out at room temperature with a constant current of 20 mA for 87 minutes, achieving the diacetoxylation reaction (Scheme 33).176
The proposed mechanism is as follows. The aromatic alkene undergoes a one-electron oxidation, resulting in the formation of a stabilized radical cation 243. This radical cation then reacts with acetate and undergoes a further one-electron oxidation to generate a benzylic cation, leading to the formation of compound 245, where the newly attached acetate group stabilizes intermediate 246. Subsequent reaction with a second acetate molecule yields the difunctionalized product 242.
This study presents an efficient electrocatalytic diacetoxylation method applicable to a wide range of alkenes, including industrial feedstock alkenes, using readily available materials and achieving yields of up to 96%. Additionally, the byproduct hydrogen gas is also a potential useful product. Notably, this is the first effective electrochemical diacetoxylation method for aliphatic alkenes such as ethylene and propylene, significantly broadening the accessibility of the resulting structural motifs. Furthermore, it has been demonstrated that aromatics can undergo diacetoxylation under catalyst-free conditions, although the yields are relatively low. In contrast to the approach of Kim's research group, this reaction does not require highly toxic solvent combinations like DCM/HFIP and DMF/TFA, highlighting its promising green, environmentally friendly, and safe characteristics. Overall, this reaction makes a valuable contribution and holds significant potential for the development of electrochemical diacetoxylation methods.
Vincent's group reported an electrochemical defunctionalization reaction of indoles involving 2,3-bis(methoxy) functionalization. They employed an undivided cell with a graphite anode and a platinum cathode, using 2,3-bismethoxylated N-acetyl skatole 247 as the model substrate. n-Bu4NBF4 served as the electrolyte, while TEMPO acted as the catalyst. A mixed solvent of methanol and acetonitrile was used. Under a constant current of 10 mA at room temperature, the reaction was successfully conducted for 2 hours to achieve the defunctionalization of 2,3-bis(methoxy) indoles (Scheme 34).177
The proposed reaction mechanism was elucidated through CV and control experiments. Initially, the N-substituted indole 247 undergoes direct oxidation at the anode to form the radical cation intermediate 249. Methoxy or azide anions generated at the cathode can react at the C3 position of 249 while oxidation occurs at the anode. This process can occur simultaneously or can involve the initial addition of a nucleophile to 249, generating a radical intermediate that is subsequently oxidized. The resulting cationic intermediate 250 is captured by another methoxy or azide molecule, ultimately leading to the formation of either 248 or 34i, as these products are more stable. In the case of 3-substituted indoles, methoxy or azide groups preferentially trans-add to avoid electronic repulsion. This reaction contrasts with the diazotization reactions developed by Lin for alkenes, where oxidative catalysis involving NaN3 transfers azide–manganese radicals that add to alkenes, followed by capture by a second azide–manganese radical.
This reaction features unique electrolytic conditions that eliminate the need for external oxidants, potentially paving the way for further developments in defunctionalization reactions. It demonstrates a broad substrate scope and compatibility with various functional groups. Furthermore, a novel mechanistic pathway is proposed, providing valuable theoretical support for the future development of electrochemical defunctionalization reactions involving 2,3-bis(methoxy) groups.
3.2. Heterodifunctionalization
In a recent breakthrough, Xu's group achieved the fluoromethylation of aryl alkenes through electrochemical methods. In a non-divided cell with a graphite rod as the anode and a platinum plate as the cathode, they used 1,1-diphenylethylene 251 and trifluoroborate 252 as model substrates, employing Et4NF·3HF as the fluorine source and acetonitrile as the solvent. The reaction was conducted at 50 °C under a constant current of 6 mA for 3 hours, successfully achieving carbon hydroxymethylation with a preference for difluorinated products (Scheme 35).178Based on variable control experiments, the proposed reaction mechanism was as follows. Alkene 251 undergoes anodic oxidation to generate the alkene radical cation 254, which is then captured by the trifluoroborate to provide the carbon-centered radical 256. Single-electron oxidation of radical 256 forms carbocation 257, which selectively reacts with the sterically less demanding nucleophile F−, yielding the final product 253. At the cathode, HF is reduced to generate H2 and F−. Since the difunctionalization reaction occurs at the anode surface, the choice of the anode material significantly impacts the reaction outcome.
This reaction demonstrates a broad substrate scope and compatibility with various functional groups, underscoring its potential as a green, environmentally friendly, and safe method. However, it has limitations, including the inability to perform gram-scale reactions, and the proposed mechanism requires further validation through experiments and DFT calculations. Overall, this study provides a convenient synthetic pathway for constructing fluorinated ethylene derivatives with good regioselectivity and chemoselectivity.
Additionally, Luo and co-workers successfully implemented the electrochemical alkoxylation of alkenes with organic halides. In a non-divided cell with a graphite rod as the anode and a platinum plate as the cathode, they used 1-methoxy-4-vinylbenzene 258, diethyl 2-bromosuccinate 259, and methanol 260 as model substrates. Tetra-n-butylammonium hydroxide (n-Bu4NOH) served as the electrolyte, Cp2Fe acted as the catalyst, and sodium hydrogen phosphate (Na2HPO4) was used as an additive, with THF as the solvent. The reaction was conducted at room temperature with a constant current density of 5 mA for 4 hours, successfully achieving the alkoxylation reaction (Scheme 36).179
The CV analysis indicated that the alkene has the lowest oxidation potential, favoring anodic oxidation. Based on detailed experiments and CV analysis, the proposed reaction mechanism was as follows. First, alkene 258 undergoes anodic oxidation to generate the corresponding radical cation 263. This is followed by an SN2 reaction, where the radical cation 263 is captured to form the carbon-centered radical 264. An alternative pathway cannot be excluded, where the brominating agent Br− is oxidized to molecular Br2, which then reacts with the radical cation 263 to generate the carbon-centered radical 264 and Br+. Subsequently, the second anodic oxidation of the carbon-centered radical 264 produces carbocation 265, which then undergoes a nucleophilic reaction with MeOH to yield the final bifunctional product 261.
This experimental method represents the first use of organic halides as halogen nucleophiles to trigger the electrochemical difunctionalization of alkenes. The reaction showcases a broad substrate scope and compatibility with various functional groups, highlighting its potential as a green, environmentally friendly, and safe approach. Moreover, it provides substantial theoretical support for further optimization of alkoxy halogenation reactions involving alkenes.
3.3. Alkene-derived radical cation-initiated rearrangement
Liu's group reported a radical cation-mediated four-component electrochemical cascade Mumm rearrangement to prepare valuable high-potential imine derivatives. In a non-divided cell with platinum plates as both the anode and cathode, they used styrene 267 and benzoic acid 268 as model substrates, with tetra-n-butylammonium hexafluorophosphate (n-Bu4NPF6) as the electrolyte. The reaction was conducted in a mixed solution of acetonitrile and methanol at room temperature at a constant current density of 5 mA for 6 hours, successfully completing the rearrangement (Scheme 37).180Based on control experiments and CV studies, the proposed reaction mechanism was as follows. Initially, styrene 267 undergoes anodic oxidation through single-electron transfer to generate the radical cation 270. This cation then undergoes nucleophilic addition with CH3O−, produced by the cathodic reduction of methanol, leading to the formation of the carbon-centered radical 272. Subsequently, anodic oxidation yields another carbocation, 273. This cation is captured by acetonitrile, resulting in carbocation 274, which can effectively combine with the PhCOO− generated from the cathodic reduction of benzoic acid. Finally, due to its structural instability, intermediate 275 quickly undergoes the Mumm rearrangement to yield the desired product 269.
This reaction demonstrates a broad substrate scope and compatibility with various functional groups, and it can be scaled to gram-level reactions. The products can be easily converted into amides and β-amino alcohols. Importantly, it also generates unstable o-acyl isocyanate precursors, which are challenging to obtain with previous methods. The approach is characterized by its low cost, the absence of oxidants and catalysts, and good scalability, underscoring its potential as a green, environmentally friendly, and safe process. Additionally, it provides a feasible method for future modular synthesis of imines.
3.4. Alkene-derived radical cation-initiated cyclization
In addition to being captured by various nucleophilic reagents, the free radical cation could also react with unsaturated hydrocarbons to form a variety of cyclic structural units, mainly including four-membered and six-membered ring derivatives. Therefore, this section mainly covers the electrochemical non-metallic catalytic oxidative cyclization of olefins and the metallic catalytic oxidative cyclization.Based on control experiments, DFT calculations, and CV studies, the proposed reaction mechanism was as follows. The aromatic radical cation 281 forms an intermolecular C–C bond with isoprene 278 through the radical cation 280 generated from the anethole at the anode, as its oxidation potential is higher than that of 277. Subsequently, the aromatic radical cation 281 can oxidize the starting trans-anethole 277 while triggering a radical cation chain mechanism, ultimately yielding the final product 279 at the cathode.
The key to successful cyclization lies in the transfer of electrons from the cyclohexene radical cation to the methoxy group. However, the use of electron-rich aryl alkenes as diene reagents is limited by the electrochemical protocol, which restricts the substrate scope and compatibility with various functional groups.
To overcome the limited range of alkenes, the group explored more practical and effective electrochemical systems. They discovered that non-conjugated alkenes can participate in radical cation-mediated Diels–Alder reactions with 1,3-dienes in non-divided cells (Scheme 38, eqn (2)),182 using a similar proposed mechanism (Scheme 38, eqn (1)).
Importantly, the reaction highlights that the use of stoichiometric or catalytic amounts of electricity is crucial for the [4 + 2] cyclization. It also showcases a broad substrate scope and compatibility with various functional groups, while being cost effective, free of oxidants and catalysts, and highly scalable, underscoring its potential as a green, environmentally friendly, and safe approach. This breakthrough discovery is expected to accelerate the development of electrochemically driven Diels–Alder reactions.
Undoubtedly, the electrochemical activation of radical cation-mediated cyclizations has significantly enriched the substrate diversity of Diels–Alder reactions. These reactions predominantly yield non-conjugated six-membered ring products via [4 + 2] cyclization. The Ye research group has described a novel electrochemical [4 + 2] cyclization involving two variants of aryl alkenes. In a non-divided cell with GF as the anode and a nickel plate as the cathode, they utilized 4-methoxy-α-methylstyrene 285 as the model substrate, with HFIP as the co-solvent, TBPA as the medium, Et4NOTs as the electrolyte, and acetonitrile as the solvent. The reaction was conducted at room temperature under a constant current of 10 mA for 2.5 hours, successfully achieving electrochemical [4 + 2] cyclization–rearrangement–aromatization (Scheme 39).183
TBPA effectively lowers the reaction potential, while HFIP, as a solvent with a high dielectric constant, stabilizes the radical or radical cation, facilitating this transformation. Notably, when the anode material was replaced with a RVC electrode, only trace amounts of the desired product were isolated. Electrolysis of dihydronaphthalene derivatives confirmed that dihydronaphthalene serves as a key intermediate for constructing naphthalene derivatives.
Further control experiments and CV studies led to the formulation of the proposed mechanism. The reaction is initiated by the anodic oxidation of TBPA, forming the stable radical cation TBPA˙+287. Then it oxidizes the electron-rich styrene 285 through SET to give styrene radical cation 288 and TBPA. As a nucleophile, 285 attacks radical cation 288 through radical cyclization to generate intermediate 289, which subsequently deprotonates to form radical 290. The loss of an electron and a proton then produces the dehydrogenated dimer 291. Similarly, this compound is oxidized by 287, resulting in the formation of the carbon radical 292, which is further oxidized to 293. Through rearrangement, a secondary carbocation transforms into a more stable tertiary carbocation 294. The electron-rich nature of the methoxy group makes it more favorable for migration compared to the methyl group. Finally, the oxidation and dehydrogenative aromatization of 294 yield the desired product 286.
The notable features of this reaction include excellent scalability and the absence of high-valent substrates, oxidants, or metals, highlighting its significant synthetic value in constructing polycyclic aromatic compounds. Importantly, mechanistic studies indicate that the dehydrogenated dimer is a critical intermediate in this process, providing valuable theoretical support for future investigations into electrochemical [4 + 2] cyclizations.
The Lei group employed a similar strategy to synthesize poly-substituted 1,2-dihydronaphthalenes. In a non-divided cell with a carbon cloth anode and a platinum plate cathode, they used α-methylstyrene 295 and the trans-anethole 296 as model substrates, with HFIP as a co-solvent and tri(4-bromophenyl) amine [(4-BrPh)3N] as the medium. Tetra-n-butylammonium perchlorate (n-Bu4NClO4) served as the electrolyte, and a mixed solvent of acetonitrile and hexafluoroisopropanol was used. Under a constant current of 7.5 mA at room temperature for 4 hours, the electrochemical [4 + 2] cyclization successfully produced poly-substituted 1,2-dihydronaphthalenes (Scheme 40).184
 | ||
| Scheme 40 Electrochemical oxidative [4 + 2] annulation of styrenes toward the synthesis of 1,2-dihydronaphthalenes. | ||
CV analysis indicated that Ar3N has a lower oxidation potential, suggesting that Ar3N is more readily oxidized than the alkene. Additionally, EPR experiments showed that this transformation can generate carbon-centered radical intermediates. A proposed reaction mechanism is as follows. Initially, (4-BrPh)3N undergoes anodic oxidation to form the nitrogen radical cation 298, which subsequently oxidizes 296 into the alkene radical cation 247 through single-electron transfer. This intermediate 299 is then attacked by the nucleophile 295, forming intermediate 300. Finally, through a series of radical cyclization, oxidation, and deprotonation steps, the desired product 297 is obtained.
This reaction also demonstrates a broad substrate scope and compatibility with various functional groups. Moreover, the approach is characterized by its low cost, the absence of oxidants and catalysts, and good scalability, underscoring its potential as a green, environmentally friendly, and safe method. Importantly, this technique provides an effective pathway for the future preparation of highly non-stereoselective dihydronaphthalenes without the use of transition metals.
The three-membered aziridine has been utilized as a precursor in medicinal chemistry and materials chemistry.185,186 However, conventional methods of aziridination, which involve hypervalent nitrogen compounds containing nitrene sources such as [N-(p-toluenesulfonyl)imino]phenyliodinane, chloramine-T, tosyl azide, and transition metal catalysis,187–192 are often overlooked in favor of epoxidation.193
Pan and co-workers reported a reaction for synthesizing quinazolinone derivatives via anodic oxidation. In a non-divided cell with a RVC anode and a platinum cathode, they utilized 2-aminobenzamide 303 and styrene 304 as model substrates, with n-Bu4NBF4 as the electrolyte, and a mixed solvent of acetonitrile and methanol. The reaction was conducted at 70 °C under a constant current of 10 mA for 12 hours, successfully achieving the cyclization (Scheme 41).194
Based on control experiments and CV studies, the proposed mechanism was as follows. First, styrene 304 undergoes anodic oxidation to form the corresponding radical cation 306, which then reacts with methanol through a 1,2-addition reaction to yield 1-(1,2-dimethoxyethyl)-4-methoxybenzene 307. Anodic re-oxidation and C–C bond cleavage of 307 generate 1-(dimethoxymethyl)-4-methoxybenzene 308. Minor amounts of water present in the solvent hydrolyze this compound to form 4-methoxybenzaldehyde 309, which then condenses with 303 to form imine 310, releasing H2O. Subsequent intramolecular cyclization yields 311. Finally, rapid anodic oxidative dehydrogenation of 311 produces the desired quinazolinone product 305. At the cathode, protons are reduced to hydrogen, completing the electrochemical cycle.
This reaction exhibits a broad substrate scope and compatibility with various functional groups. The approach is characterized by its low cost, the absence of oxidants and catalysts, and good scalability, underscoring its potential as a green, environmentally friendly, and safe method. Importantly, the research group also investigated the effect of internal alkenes on reaction activity and found that internal alkenes are also suitable for this reaction. For asymmetric alkenes, the yield of electron-rich aromatics is higher than that of electron-deficient ones. Compared to terminal alkenes, internal alkenes exhibit a lower reaction rate due to steric hindrance. This work provides a solid theoretical foundation for further research into the synthesis of other functional heterocyclic products.
Based on control experiments and CV studies, the proposed mechanism was as follows. The anodic oxidation of indole 313 generates the indole radical cation intermediate 315. Under the basic conditions of the NaOTf/n-Bu4NI mixed electrolyte, deprotonation occurs sequentially, forming the indole carbon-centered radical intermediate 316. In the Co–alkene complex 317, radical intermediate 316 adds to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, generating the radical alkyl intermediate 318, which is subsequently oxidized to the alkyl cation intermediate 319 by anodic single-electron oxidation. Another molecule of indole 313 undergoes electrophilic alkylation with intermediate 319, yielding the indole cation intermediate 320. Protonation occurs at the platinum cathode to produce the desired dinitrogen product 321. When using graphite rods as both anode and cathode, intermediate 320 undergoes intramolecular electrophilic alkylation to form intermediate 322. The low redox potential between the graphite rod anode and cathode leads to slower electron transfer, necessitating a stronger constant current. When replacing the platinum cathode with a graphite rod cathode, the overpotential for proton reduction increases, making it difficult for intermediate 320 to lose a proton and generate product 321. Additionally, the high surface area of the porous carbon material facilitates the adsorption and further oxidation of intermediate 320 rather than diffusion. Ultimately, continuous anodic single-electron oxidation and deprotonation yield the dehydrogenated [2 + 2 + 2] cycloadduct 314.
C bond, generating the radical alkyl intermediate 318, which is subsequently oxidized to the alkyl cation intermediate 319 by anodic single-electron oxidation. Another molecule of indole 313 undergoes electrophilic alkylation with intermediate 319, yielding the indole cation intermediate 320. Protonation occurs at the platinum cathode to produce the desired dinitrogen product 321. When using graphite rods as both anode and cathode, intermediate 320 undergoes intramolecular electrophilic alkylation to form intermediate 322. The low redox potential between the graphite rod anode and cathode leads to slower electron transfer, necessitating a stronger constant current. When replacing the platinum cathode with a graphite rod cathode, the overpotential for proton reduction increases, making it difficult for intermediate 320 to lose a proton and generate product 321. Additionally, the high surface area of the porous carbon material facilitates the adsorption and further oxidation of intermediate 320 rather than diffusion. Ultimately, continuous anodic single-electron oxidation and deprotonation yield the dehydrogenated [2 + 2 + 2] cycloadduct 314.
This reaction showcases a broad substrate scope and compatibility with various functional groups. The approach is characterized by its low cost, the absence of oxidants and catalytic conditions, and good scalability, highlighting its potential as a green, environmentally friendly, and safe method. Notably, the innovative use of a graphite rod cathode instead of a platinum cathode establishes an unprecedented dehydrogenative [2 + 2 + 2] cycloaddition reaction between alkenes and indoles through an electrochemical radical relay strategy. Importantly, this method demonstrates multifunctional potential for the late-stage modification of valuable bioactive molecules.
4. Alkenes as radical anion intermediates
Both direct activation of nucleophiles to generate free radical intermediates and direct activation of olefins to form the corresponding radical cations prior to bielectrophilic transformations have been realized, with many successful examples demonstrating their feasibility.31 These strategies relied on oxidation activation of reactants or catalysts and were often limited by the requirements for strong nucleophilic reagents, easily oxidizable olefins, and stable radical cations.196In contrast, radical anions derived from conjugated alkenes exhibit strong nucleophilicity.197 The development of methods utilizing alkenes as radical anion reaction intermediates has opened up new strategies for their difunctionalization. By directly generating the corresponding radical anions through single-electron reduction, weak nucleophilic alkenes can be activated, providing an orthogonal reaction mode for the functionalization of C–C double bonds with weak electrophilic reagents. Furthermore, cathodic electro-reduction is safer than traditional reduction methods, as it avoids the use of hazardous chemical reducing agents such as metal hydrides, boranes, and silanes.
However, enhancing the efficiency and selectivity of these reactions to achieve optimal product outcomes remains a challenge. Furthermore, the use of toxic heavy metals in cathode materials may pose significant health risks. Therefore, the wise selection of suitable cathode materials is a key factor for the successful implementation of electro-cathodic reactions. Despite these obstacles, significant progress has been made in cathodic electroreduction.198
Fortunately, Buckley and his colleagues have reported an electrochemical method for the selective α,δ-dicarboxylation of conjugated dienes using carbon dioxide. They employed 1-phenyl-1,3-butadiene 323 as the model substrate, with n-Bu4NI as the electrolyte, in a non-divided cell with carbon as the anode and stainless steel as the cathode. The reaction was conducted at room temperature under a CO2 atmosphere in a DMF and water solution, at a constant voltage of 10 V for 4 hours, successfully achieving α,δ-dicarboxylation (Scheme 43).199
The proposed mechanism includes the following steps. Hydroxide ions lose electrons to form oxygen and protons, after which electrons are transferred to the diene, generating a radical anion adsorbed on the diene 325. Finally, further electron transfer and protonation of the protons result in dicarboxylation.
Although this reaction only produces δ-monocarboxylic olefins as a byproduct and requires conditions with very minimal or no proton sources such as TEOA and H2O to achieve high yields, it represents a crucial first step in exploring the use of olefins as radical anion intermediates for achieving difunctionalization. This is a significant advancement in the field.
5. Summary and outlook
In recent years, radical-mediated difunctionalization of alkenes has made significant progress in the field of electroorganic synthesis, opening up promising avenues for the development of efficient and sustainable synthetic methods in organic chemistry. Research has shown that electrochemical methods exhibit great potential for achieving precise and selective difunctionalization of alkenes. The notable advantages of these methods include the use of mild reaction conditions, the avoidance of toxic reagents, and the ability to control reactions through electrical parameters, which provides new pathways for synthesizing various functionalized compounds.In the days to come, several essential areas warrant in-depth investigation. First, it is crucial to develop more efficient and stable electrode materials. New electrode designs can enhance electron transfer rates, improve selectivity, and reduce overpotential, making electrochemical processes more energy-efficient and economically viable. Second, a deeper understanding of reaction mechanisms is needed to better control reaction pathways and design more targeted and effective synthetic strategies. This includes clarifying the role of intermediates and their effects on regioselectivity and stereoselectivity. Additionally, integrating electroorganic synthesis with emerging technologies such as flow chemistry and microreaction systems could significantly enhance the scalability and productivity of processes, potentially transforming the industrial application of alkene difunctionalization reactions. From an environmental perspective, developing green and sustainable electrolytes is vital. Research into biodegradable or recyclable electrolytes will help minimize the environmental impact of electrochemical processes. Finally, the potential of electroorganic synthesis in the synthesis of complex natural products and pharmaceuticals is substantial; by providing alternative pathways for constructing key structural motifs, this method can make significant contributions to drug discovery and materials science.
In summary, radical-mediated difunctionalization of alkenes through electroorganic synthesis is an exciting and rapidly evolving field. Continued research and innovation in these areas are expected to yield further breakthroughs, providing efficient and sustainable solutions for the synthesis of valuable organic compounds and advancing the field of organic chemistry.
Data availability
Data availability is not applicable to this article as no new data were created or analyzed in this study.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by National Natural Science Foundation of China (NSFC, Grant No. 52401244, 52201227, 62204246 and 62341402), the Zhejiang Provincial Natural Science Foundation of China (Grant No. Q24B020025), and the Wenzhou Basic Scientific Research Project (G20220020).References
- H. Wu and K. D. Moeller, Org. Lett., 2007, 9, 4599–4602 CrossRef CAS PubMed.
- M. Bittner, J. Jakupovic, F. Bohlmann and M. Silva, Phytochemistry, 1989, 28, 1887–1890 CrossRef CAS.
- E. N. Thobokholt, E. L. Larghi, A. B. Bracca and T. S. Kaufman, RSC Adv., 2020, 10, 18978–19002 RSC.
- E. Steckhan, T. Arns, W. R. Heineman, G. Hilt, D. Hoormann, J. Jörissen, L. Kröner, B. Lewall and H. Pütter, Chemosphere, 2001, 43, 63–73 CrossRef CAS PubMed.
- B. A. Frontana-Uribe, R. D. Little, J. G. Ibanez, A. Palma and R. Vasquez-Medrano, Green Chem., 2010, 12, 2099–2119 RSC.
- R. Francke and R. D. Little, Chem. Soc. Rev., 2014, 43, 2492–2521 RSC.
- E. V. LaBelle, C. W. Marshall and H. D. May, Acc. Chem. Res., 2019, 53, 62–71 CrossRef PubMed.
- Y. Zhang, X. Zhao and G. Qing, Chem. Synth., 2024, 4, 16 CAS.
- J. H. Qin, N. Nan and J. H. Li, Synthesis, 2023, 2843–2859 CrossRef CAS.
- G. M. Martins, B. Shirinfar, T. Hardwick and N. Ahmed, ChemElectroChem, 2019, 6, 1300–1315 CrossRef CAS.
- G. S. Sauer and S. Lin, ACS Catal., 2018, 8, 5175–5187 CrossRef CAS.
- F. Wang, P. Chen and G. Liu, Acc. Chem. Res., 2018, 51, 2036–2046 CrossRef CAS PubMed.
- J. Lin, R. J. Song, M. Hu and J. H. Li, Chem. Rec., 2019, 19, 440–451 CrossRef CAS PubMed.
- L. F. Novaes, J. Liu, Y. Shen, L. Lu, J. M. Meinhardt and S. Lin, Chem. Soc. Rev., 2021, 50, 7941–8002 RSC.
- H. Jiang and A. Studer, Chem. Soc. Rev., 2020, 49, 1790–1811 RSC.
- T. Xiong and Q. Zhang, Chem. Soc. Rev., 2021, 50, 8857–8873 RSC.
- J. D. Brandt and K. D. Moeller, Org. Lett., 2005, 7, 3553–3556 CrossRef CAS PubMed.
- G. Xu and K. D. Moeller, Org. Lett., 2010, 12, 2590–2593 CrossRef CAS PubMed.
- Z. Xiang, J. Mol. Struct., 2012, 1029, 15–21 CrossRef CAS.
- K. A. Margrey and D. A. Nicewicz, Acc. Chem. Res., 2016, 49, 1997–2006 CrossRef CAS PubMed.
- R. Feng, J. A. Smith and K. D. Moeller, Acc. Chem. Res., 2017, 50, 2346–2352 CrossRef CAS PubMed.
- Y. Li, R. Lu, S. Sun and L. Liu, Org. Lett., 2018, 20, 6836–6839 CrossRef CAS PubMed.
- J. H. Shin, E. Y. Seong, H. J. Mun, Y. J. Jang and E. J. Kang, Org. Lett., 2018, 20, 5872–5876 CrossRef CAS PubMed.
- Y. H. Cho, J. H. Kim, H. An, K. H. Ahn and E. J. Kang, Adv. Synth. Catal., 2020, 362, 2183–2188 CrossRef.
- M. Patel, B. Desai, A. Sheth, B. Z. Dholakiya and T. Naveen, Asian J. Org. Chem., 2021, 10, 3201–3232 CrossRef CAS.
- A. L. Gant Kanegusuku and J. L. Roizen, Angew. Chem., Int. Ed., 2021, 60, 21116–21149 CrossRef CAS PubMed.
- N. Chen and H. C. Xu, Chem. Rec., 2021, 21, 2306–2319 CrossRef CAS PubMed.
- J. Dong, F. Yue, J. Liu, H. Song, Y. Liu and Q. Wang, Green Chem., 2021, 23, 7963–7968 RSC.
- X. Liu, R. Liu, J. Qiu, X. Cheng and G. Li, Angew. Chem., Int. Ed., 2020, 59, 13962–13967 CrossRef CAS PubMed.
- H. Huang, J.-H. Ye, L. Zhu, C.-K. Ran, M. Miao, W. Wang, H. Chen, W.-J. Zhou, Y. Lan and B. Yu, CCS Chem., 2021, 3, 1746–1756 CrossRef CAS.
- M. L. Czyz, M. S. Taylor, T. H. Horngren and A. Polyzos, ACS Catal., 2021, 11, 5472–5480 CrossRef CAS.
- H. Mei, Z. Yin, J. Liu, H. Sun and J. Han, Chin. J. Chem., 2019, 37, 292–301 CrossRef CAS.
- D. A. Thadathil, A. Varghese and K. V. Radhakrishnan, Asian J. Org. Chem., 2021, 10, 2820–2847 CrossRef CAS.
- Y. A. Yuan, D. F. Lu, Y. R. Chen and H. Xu, Angew. Chem., 2016, 128, 544–548 CrossRef.
- W. E. Fristad, T. A. Brandvold, J. R. Peterson and S. R. Thompson, J. Org. Chem., 1985, 50, 3647–3649 CrossRef CAS.
- D. Lucet, T. Le Gall and C. Mioskowski, Angew. Chem., Int. Ed., 1998, 37, 2580–2627 CrossRef CAS PubMed.
- Y. L. Bennani and S. Hanessian, Chem. Rev., 1997, 97, 3161–3196 CrossRef CAS PubMed.
- F. Fache, E. Schulz, M. L. Tommasino and M. Lemaire, Chem. Rev., 2000, 100, 2159–2232 CrossRef CAS PubMed.
- E. Bogatcheva, C. Hanrahan, B. Nikonenko, R. Samala, P. Chen, J. Gearhart, F. Barbosa, L. Einck, C. A. Nacy and M. Protopopova, J. Med. Chem., 2006, 49, 3045–3048 CrossRef CAS PubMed.
- J.-C. Kizirian, Chem. Rev., 2008, 108, 140–205 CrossRef CAS PubMed.
- J. González-Sabín, F. Rebolledo and V. Gotor, Chem. Soc. Rev., 2009, 38, 1916–1925 RSC.
- O. O. Grygorenko, D. S. Radchenko, D. M. Volochnyuk, A. A. Tolmachev and I. V. Komarov, Chem. Rev., 2011, 111, 5506–5568 CrossRef CAS PubMed.
- B. Zhang and A. Studer, Org. Lett., 2014, 16, 1790–1793 CrossRef CAS PubMed.
- N. Fu, G. S. Sauer, A. Saha, A. Loo and S. Lin, Science, 2017, 357, 575–579 CrossRef CAS PubMed.
- J. C. Siu, J. B. Parry and S. Lin, J. Am. Chem. Soc., 2019, 141, 2825–2831 CrossRef CAS PubMed.
- C.-Y. Cai, Y.-T. Zheng, J.-F. Li and H.-C. Xu, J. Am. Chem. Soc., 2022, 144, 11980–11985 CrossRef CAS PubMed.
- P. D. Magnus, Tetrahedron Lett., 1977, 33, 2019–2045 CrossRef CAS.
- D. C. Meadows and J. Gervay-Hague, Med. Res. Rev., 2006, 26, 793–814 CrossRef CAS PubMed.
- J. Aziz, S. Messaoudi, M. Alami and A. Hamze, Org. Biomol. Chem., 2014, 12, 9743–9759 RSC.
- N.-W. Liu, S. Liang and G. Manolikakes, Synthesis, 2016, 1939–1973 CAS.
- A. Abouimrane, I. Belharouak and K. Amine, Electrochem. Commun., 2009, 11, 1073–1076 CrossRef CAS.
- J. J. Caldwell and D. Craig, Angew. Chem., 2007, 119, 2685–2688 CrossRef.
- Z.-H. Wen, C.-H. Chao, M.-H. Wu and J.-H. Sheu, Eur. J. Med. Chem., 2010, 45, 5998–6004 CrossRef CAS PubMed.
- D. Braghiroli, R. Avallone and M. Di Bella, Tetrahedron: Asymmetry, 1997, 8, 2209–2213 CrossRef CAS.
- H. Nakamura, H. Wu, J. Kobayashi, M. Kobayashi, Y. Ohizumi and Y. Hirata, J. Org. Chem., 1985, 50, 2494–2497 CrossRef CAS.
- S. Oida, Y. Tajima, T. Konosu, Y. Nakamura, A. Somada, T. Tanaka, S. Habuki, T. Harasaki, Y. Kamai and T. Fukuoka, Chem. Pharm. Bull., 2000, 48, 694–707 CrossRef CAS PubMed.
- A. Guerrini, A. Tesei, C. Ferroni, G. Paganelli, A. Zamagni, S. Carloni, M. Di Donato, G. Castoria, C. Leonetti and M. Porru, J. Med. Chem., 2014, 57, 7263–7279 CrossRef CAS PubMed.
- H. Mei, J. Liu, Y. Guo and J. Han, ACS Omega, 2019, 4, 14353–14359 CrossRef CAS PubMed.
- Z. Zhang, J. Yan, D. Ma and J. Sun, Chin. Chem. Lett., 2019, 30, 1509–1511 CrossRef CAS.
- Y. Wang, L. Deng, H. Mei, B. Du, J. Han and Y. Pan, Green Chem., 2018, 20, 3444–3449 RSC.
- J.-L. Wan and J.-M. Huang, Org. Lett., 2022, 24, 8914–8919 CrossRef CAS PubMed.
- M. G. Dolson and J. S. Swenton, J. Am. Chem. Soc., 1981, 103, 2361–2371 CrossRef CAS.
- T. Sumi, T. Saitoh, K. Natsui, T. Yamamoto, M. Atobe, Y. Einaga and S. Nishiyama, Angew. Chem., Int. Ed., 2012, 51, 5443–5446 CrossRef CAS PubMed.
- A. Kirste, B. Elsler, G. Schnakenburg and S. R. Waldvogel, J. Am. Chem. Soc., 2012, 134, 3571–3576 CrossRef CAS PubMed.
- V. Quint, F. Morlet-Savary, J.-F. Lohier, J. Lalevee, A.-C. Gaumont and S. Lakhdar, J. Am. Chem. Soc., 2016, 138, 7436–7441 CrossRef CAS PubMed.
- K. Liang, Q. Zhang and C. Guo, Sci. Adv., 2022, 8, eadd7134 CrossRef CAS PubMed.
- R. Wang, N. Zhang, Y. Zhang, B. Wang, Y. Xia, K. Sun, W. Jin, X. Li and C. Liu, Green Chem., 2023, 25, 3925–3930 RSC.
- S. H. Park, J. Jang, K. Shin and H. Kim, ACS Catal., 2022, 12, 10572–10580 CrossRef CAS.
- M. G. Campbell and T. Ritter, Chem. Rev., 2015, 115, 612–633 CrossRef CAS PubMed.
- B. Lantaño and A. Postigo, Org. Biomol. Chem., 2017, 15, 9954–9973 RSC.
- L. Yang, T. Dong, H. M. Revankar and C.-P. Zhang, Green Chem., 2017, 19, 3951–3992 RSC.
- P. J. Monsen and F. A. Luzzio, ARKIVOC, 2017, 117–147 Search PubMed.
- P. Richardson, Expert Opin. Drug Delivery, 2016, 11, 983–999 Search PubMed.
- D. E. Yerien, S. Bonesi and A. Postigo, Org. Biomol. Chem., 2016, 14, 8398–8427 RSC.
- P. A. Champagne, J. Desroches, J.-D. Hamel, M. Vandamme and J.-F. Paquin, Chem. Rev., 2015, 115, 9073–9174 CrossRef CAS PubMed.
- J.-P. Bégué and D. Bonnet-Delpon, Bioorganic and medicinal chemistry of fluorine, John Wiley & Sons, 2008 Search PubMed.
- T. Hiyama, T. Hiyama and H. Yamamoto, Organofluorine Compounds: Chemistry and Applications, 2000, pp. 137–182 Search PubMed.
- T. Yamazaki, T. Taguchi and I. Ojima, Fluorine in medicinal chemistry and chemical biology, 2009, vol. 1 Search PubMed.
- P. Jeschke, ChemBioChem, 2004, 5, 570–589 CrossRef CAS PubMed.
- K. Muller, C. Faeh and F. Diederich, Science, 2007, 317, 1881–1886 CrossRef PubMed.
- J.-A. Ma and D. Cahard, Chem. Rev., 2004, 104, 6119–6146 CrossRef CAS PubMed.
- J.-A. Ma and D. Cahard, J. Fluorine Chem., 2007, 128, 975–996 CrossRef CAS.
- I. Omae, Appl. Organomet. Chem., 2008, 22, 149–166 CrossRef CAS.
- T. Furuya, A. S. Kamlet and T. Ritter, Nature, 2011, 473, 470–477 CrossRef CAS PubMed.
- A. G. O'Brien, A. Maruyama, Y. Inokuma, M. Fujita, P. S. Baran and D. G. Blackmond, Angew. Chem., Int. Ed., 2014, 53, 11868–11871 CrossRef PubMed.
- H. Liu, Z. Gu and X. Jiang, Adv. Synth. Catal., 2013, 355, 617–626 CrossRef CAS.
- T. Besset, C. Schneider and D. Cahard, Angew. Chem., Int. Ed., 2012, 21, 5134–5136 CrossRef.
- C. Alonso, E. Martinez de Marigorta, G. Rubiales and F. Palacios, Chem. Rev., 2015, 115, 1847–1935 CrossRef CAS PubMed.
- Y. Li and A. Studer, Angew. Chem., 2012, 124, 8345–8348 CrossRef.
- Y. Yasu, T. Koike and M. Akita, Angew. Chem., 2012, 51, 9567–9571 CrossRef CAS PubMed.
- X. Y. Jiang and F. L. Qing, Angew. Chem., 2013, 125, 14427–14430 CrossRef.
- M. Yan, Y. Kawamata and P. S. Baran, Chem. Rev., 2017, 117, 13230–13319 CrossRef CAS PubMed.
- P.-G. Echeverria, D. Delbrayelle, A. Letort, F. Nomertin, M. Perez and L. Petit, Aldrichimica Acta, 2018, 51, 3–19 Search PubMed.
- A. Wiebe, T. Gieshoff, S. Möhle, E. Rodrigo, M. Zirbes and S. R. Waldvogel, Angew. Chem., 2018, 130, 5694–5721 CrossRef.
- S. R. Waldvogel, S. Lips, M. Selt, B. Riehl and C. J. Kampf, Chem. Rev., 2018, 118, 6706–6765 CrossRef CAS PubMed.
- S. Möhle, M. Zirbes, E. Rodrigo, T. Gieshoff, A. Wiebe and S. R. Waldvogel, Angew. Chem., Int. Ed., 2018, 57, 6018–6041 CrossRef PubMed.
- J.-I. Yoshida, A. Shimizu and R. Hayashi, Chem. Rev., 2017, 118, 4702–4730 CrossRef PubMed.
- M. Yan, Y. Kawamata and P. S. Baran, Angew. Chem., 2018, 130, 4219–4225 CrossRef.
- E. Horn, B. Rosen and P. Baran, Science, 2016, 2, 302–308 CAS.
- W. Jud, C. O. Kappe and D. Cantillo, Chem. – Eur. J., 2018, 24, 17234–17238 CrossRef CAS PubMed.
- K.-Y. Ye, G. Pombar, N. Fu, G. S. Sauer, I. Keresztes and S. Lin, J. Am. Chem. Soc., 2018, 140, 2438–2441 CrossRef CAS PubMed.
- B. B. Snider and H. Lin, Synth. Commun., 1998, 28, 1913–1922 CrossRef CAS.
- B. B. Snider and S. M. O'Hare, Synth. Commun., 2001, 31, 3753–3758 CrossRef CAS.
- F. F. Fleming, Nat. Prod. Rep., 1999, 16, 597–606 RSC.
- F. F. Fleming, L. Yao, P. Ravikumar, L. Funk and B. C. Shook, J. Med. Chem., 2010, 53, 7902–7917 CrossRef CAS PubMed.
- F. F. Fleming and Q. Wang, Chem. Rev., 2003, 103, 2035–2078 CrossRef CAS PubMed.
- M. Ishikura, T. Abe, T. Choshi and S. Hibino, Nat. Prod. Rep., 2015, 32, 1389–1471 RSC.
- N. Fu, L. Song, J. Liu, Y. Shen, J. C. Siu and S. Lin, J. Am. Chem. Soc., 2019, 141, 14480–14485 CrossRef CAS PubMed.
- X. Kong, X. Chen, Y. Chen and Z.-Y. Cao, J. Org. Chem., 2022, 87, 7013–7021 CrossRef CAS PubMed.
- N. Fu, Y. Shen, A. R. Allen, L. Song, A. Ozaki and S. Lin, ACS Catal., 2018, 9, 746–754 CrossRef PubMed.
- H. H. Xu, J. Song and H. C. Xu, ChemSusChem, 2019, 12, 3060–3063 CrossRef CAS PubMed.
- C. Dai, Y. Shen, Y. Wei, P. Liu and P. Sun, J. Org. Chem., 2021, 86, 13711–13719 CrossRef CAS PubMed.
- W. Li, W. Xu, J. Xie, S. Yu and C. Zhu, Chem. Soc. Rev., 2018, 47, 654–667 RSC.
- X. Wu, S. Wu and C. Zhu, Tetrahedron Lett., 2018, 59, 1328–1336 CrossRef CAS.
- Z.-M. Chen, X.-M. Zhang and Y.-Q. Tu, Chem. Soc. Rev., 2015, 44, 5220–5245 RSC.
- A. Studer and M. Bossart, Tetrahedron Lett., 2001, 57, 9649–9667 CrossRef CAS.
- Y. Zeng, C. Ni and J. Hu, Chem. – Eur. J., 2016, 22, 3210–3223 CrossRef CAS PubMed.
- Y. Li, B. Liu, H.-B. Li, Q. Wang and J.-H. Li, Chem. Commun., 2015, 51, 1024–1026 RSC.
- H.-L. Huang, H. Yan, C. Yang and W. Xia, Chem. Commun., 2015, 51, 4910–4913 RSC.
- J. Zhao, H. Fang, R. Song, J. Zhou, J. Han and Y. Pan, Chem. Commun., 2015, 51, 599–602 RSC.
- L. Zheng, C. Yang, Z. Xu, F. Gao and W. Xia, J. Org. Chem., 2015, 80, 5730–5736 CrossRef CAS PubMed.
- W. Thaharn, D. Soorukram, C. Kuhakarn, P. Tuchinda, V. Reutrakul and M. Pohmakotr, Angew. Chem., Int. Ed., 2014, 53, 2212–2215 CrossRef CAS PubMed.
- W. Kong, M. Casimiro, E. B. Merino and C. Nevado, J. Am. Chem. Soc., 2013, 135, 14480–14483 CrossRef CAS PubMed.
- W. Kong, E. Merino and C. Nevado, Angew. Chem., Int. Ed., 2014, 53, 5078–5082 CrossRef CAS PubMed.
- Z. Zou, W. Zhang, Y. Wang, L. Kong, G. Karotsis, Y. Wang and Y. Pan, Org. Lett., 2019, 21, 1857–1862 CrossRef CAS PubMed.
- J.-C. Kang, Y.-Q. Tu, J.-W. Dong, C. Chen, J. Zhou, T.-M. Ding, J.-T. Zai, Z.-M. Chen and S.-Y. Zhang, Org. Lett., 2019, 21, 2536–2540 CrossRef CAS PubMed.
- Y. Gao, H. Mei, J. Han and Y. Pan, Chem. – Eur. J., 2018, 24, 17205–17209 CrossRef CAS PubMed.
- M. Chen, Z. J. Wu, J. Song and H. C. Xu, Angew. Chem., Int. Ed., 2022, 61, e202115954 CrossRef CAS PubMed.
- F. Arshad, M. F. Khan, W. Akhtar, M. M. Alam, L. M. Nainwal, S. K. Kaushik, M. Akhter, S. Parvez, S. M. Hasan and M. Shaquiquzzaman, Eur. J. Med. Chem., 2019, 167, 324–356 CrossRef CAS PubMed.
- M. Andrs, J. Korabecny, D. Jun, Z. Hodny, J. Bartek and K. Kuca, J. Med. Chem., 2015, 58, 41–71 CrossRef CAS PubMed.
- E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257–10274 CrossRef CAS PubMed.
- A. Y. Rulev, A. R. Romanov, E. V. Kondrashov, I. A. Ushakov, V. M. Muzalevskiy and V. G. Nenajdenko, Eur. J. Med. Chem., 2018, 2018, 4202–4210 CAS.
- S. Kenis, M. D′hooghe, G. Verniest, M. Reybroeck, T. A. Dang Thi, C. Pham The, T. Thi Pham, K. W. Törnroos, N. Van Tuyen and N. De Kimpe, Chem. – Eur. J., 2013, 19, 5966–5971 CrossRef CAS PubMed.
- T. Takata, K. Hirano and M. Miura, Org. Lett., 2019, 21, 4284–4288 CrossRef CAS PubMed.
- A. Claraz, T. Courant and G. Masson, Org. Lett., 2020, 22, 1580–1584 CrossRef CAS PubMed.
- Z. Li, L. Jiao, Y. Sun, Z. He, Z. Wei and W. W. Liao, Angew. Chem., 2020, 132, 7333–7337 CrossRef.
- Y.-Y. Jiang, G.-Y. Dou, K. Xu and C.-C. Zeng, Org. Chem. Front., 2018, 5, 2573–2577 RSC.
- N. Ahmed and S. Khatoon, ChemistryOpen, 2018, 7, 576–582 CrossRef CAS PubMed.
- Y. Zhao, X. Li, S. L. Homölle, B. Wang and L. Ackermann, Chem. Sci., 2024, 15, 1117–1122 RSC.
- C. Song, K. Liu, X. Jiang, X. Dong, Y. Weng, C. W. Chiang and A. Lei, Angew. Chem., Int. Ed., 2020, 59, 7193–7197 CrossRef CAS PubMed.
- M.-X. He, Z.-Y. Mo, Z.-Q. Wang, S.-Y. Cheng, R.-R. Xie, H.-T. Tang and Y.-M. Pan, Org. Lett., 2019, 22, 724–728 CrossRef PubMed.
- J. H. Horner and M. Newcomb, J. Org. Chem., 2007, 72, 1609–1616 CrossRef CAS PubMed.
- N. A. Romero and D. A. Nicewicz, J. Am. Chem. Soc., 2014, 136, 17024–17035 CrossRef CAS PubMed.
- C. Ma, P. Fang and T. S. Mei, ACS Catal., 2018, 8, 7179–7189 CrossRef CAS.
- P. Xiong and H.-C. Xu, Acc. Chem. Res., 2019, 52, 3339–3350 CrossRef CAS PubMed.
- Y. Yuan and A. Lei, Acc. Chem. Res., 2019, 52, 3309–3324 CrossRef CAS PubMed.
- J. L. Röckl, D. Pollok, R. Franke and S. R. Waldvogel, Acc. Chem. Res., 2019, 53, 45–61 CrossRef PubMed.
- L. Ackermann, Acc. Chem. Res., 2019, 53, 84–104 CrossRef PubMed.
- K. Yamamoto, M. Kuriyama and O. Onomura, Acc. Chem. Res., 2019, 53, 105–120 CrossRef PubMed.
- P. Qian, Z. Zha and Z. Wang, ChemElectroChem, 2020, 7, 2527–2544 CrossRef CAS.
- D. M. Heard and A. J. Lennox, Angew. Chem., Int. Ed., 2020, 59, 18866–18884 CrossRef CAS PubMed.
- J. Liu, L. Lu, D. Wood and S. Lin, ACS Cent. Sci., 2020, 6, 1317–1340 CrossRef CAS PubMed.
- S.-H. Shi, Y. Liang and N. Jiao, Chem. Rev., 2020, 121, 485–505 CrossRef PubMed.
- Y. Yuan, J. Yang and A. Lei, Chem. Soc. Rev., 2021, 50, 10058–10086 RSC.
- E. Raoult, J. Sarrazin and A. Tallec, J. Appl. Electrochem., 1984, 14, 639–643 CrossRef CAS.
- S. M. Halas, K. Okyne and A. J. Fry, Electrochim. Acta, 2003, 48, 1837–1844 CrossRef CAS.
- E. Steckhan, Angew. Chem., Int. Ed. Engl., 1986, 25, 683–701 CrossRef.
- A. Badalyan and S. S. Stahl, Nature, 2016, 535, 406–410 CrossRef CAS PubMed.
- J. E. Nutting, M. Rafiee and S. S. Stahl, Chem. Rev., 2018, 118, 4834–4885 CrossRef CAS PubMed.
- Z. W. Hou, Z. Y. Mao, Y. Y. Melcamu, X. Lu and H. C. Xu, Angew. Chem., Int. Ed., 2018, 57, 1636–1639 CrossRef CAS PubMed.
- M. Rafiee, F. Wang, D. P. Hruszkewycz and S. S. Stahl, J. Am. Chem. Soc., 2018, 140, 22–25 CrossRef CAS PubMed.
- X. Bao, J. Li, W. Jiang and C. Huo, Synthesis, 2019, 4507–4530 CAS.
- Y. C. Wu, Y. T. Xiao, Y. Z. Yang, R. J. Song and J. H. Li, ChemCatChem, 2020, 12, 5312–5329 CrossRef CAS.
- X. Wu and C. Zhu, Acc. Chem. Res., 2020, 53, 1620–1636 CrossRef CAS PubMed.
- C.-Y. Cai, X.-M. Shu and H.-C. Xu, Nat. Commun., 2019, 10, 4953 CrossRef CAS PubMed.
- B. Belleau and Y. Au-Young, Can. J. Chem., 1969, 47, 2117–2118 CrossRef CAS.
- Y. Li, D. Song and V. M. Dong, J. Am. Chem. Soc., 2008, 130, 2962–2964 CrossRef CAS PubMed.
- J. Seayad, A. M. Seayad and C. L. Chai, Org. Lett., 2010, 12, 1412–1415 CrossRef CAS PubMed.
- B. C. Giglio, V. A. Schmidt and E. J. Alexanian, J. Am. Chem. Soc., 2011, 133, 13320–13322 CrossRef CAS PubMed.
- W. Zhong, S. Liu, J. Yang, X. Meng and Z. Li, Org. Lett., 2012, 14, 3336–3339 CrossRef CAS PubMed.
- S. Picon, M. Rawling, M. Campbell and N. C. Tomkinson, Org. Lett., 2012, 14, 6250–6253 CrossRef CAS PubMed.
- Z. K. Wickens, P. E. Guzmán and R. H. Grubbs, Angew. Chem., Int. Ed., 2015, 54, 236–240 CrossRef CAS PubMed.
- Y. B. Kang, X. M. Chen, C. Z. Yao and X. S. Ning, Chem. Commun., 2016, 52, 6193–6196 RSC.
- S. Zhang, L. Li, P. Wu, P. Gong, R. Liu and K. Xu, Adv. Synth. Catal., 2019, 361, 485–489 CrossRef CAS.
- D. S. Chung, S. H. Park, S.-G. Lee and H. Kim, Chem. Sci., 2021, 12, 5892–5897 RSC.
- J. R. Vanhoof, P. J. De Smedt, J. Derhaeg, R. Ameloot and D. E. De Vos, Angew. Chem., 2023, 135, e202311539 CrossRef.
- J. Wu, Y. Dou, R. Guillot, C. Kouklovsky and G. Vincent, J. Am. Chem. Soc., 2019, 141, 2832–2837 CrossRef CAS PubMed.
- P. Xiong, H. Long and H. C. Xu, Asian J. Org. Chem., 2019, 8, 658–660 CrossRef CAS.
- T.-T. Zhang, M.-J. Luo, Y. Li, R.-J. Song and J.-H. Li, Org. Lett., 2020, 22, 7250–7254 CrossRef CAS PubMed.
- X. Zhang, T. Cui, X. Zhao, P. Liu and P. Sun, Angew. Chem., 2020, 132, 3493–3497 CrossRef.
- Y. Okada, Y. Yamaguchi, A. Ozaki and K. Chiba, Chem. Sci., 2016, 7, 6387–6393 RSC.
- A. Ozaki, Y. Yamaguchi, Y. Okada and K. Chiba, Chin. J. Chem., 2019, 37, 561–564 CrossRef CAS.
- Y. Ma, J. Lv, C. Liu, X. Yao, G. Yan, W. Yu and J. Ye, Angew. Chem., Int. Ed., 2019, 58, 6756–6760 CrossRef CAS PubMed.
- K. Liang, S. Wang, H. Cong, L. Lu and A. Lei, CCS Chem., 2022, 4, 1557–1564 CrossRef CAS.
- J. L. Jat, M. P. Paudyal, H. Gao, Q.-L. Xu, M. Yousufuddin, D. Devarajan, D. H. Ess, L. Kürti and J. R. Falck, Science, 2014, 343, 61–65 CrossRef CAS PubMed.
- O. Dogan and S. Polat-Cakir, Chem. Heterocycl. Compd., 2018, 54, 397–399 CrossRef CAS.
- L. Simkhovich and Z. Gross, Tetrahedron Lett., 2001, 42, 8089–8092 CrossRef CAS.
- J. Du Bois, C. S. Tomooka, J. Hong and E. M. Carreira, Acc. Chem. Res., 1997, 30, 364–372 CrossRef CAS.
- G.-Y. Gao, J. D. Harden and X. P. Zhang, Org. Lett., 2005, 7, 3191–3193 CrossRef CAS PubMed.
- S. L. Jain, V. B. Sharma and B. Sain, Tetrahedron Lett., 2004, 45, 8731–8732 CrossRef CAS.
- M. A. Mairena, M. M. Díaz-Requejo, T. R. Belderraín, M. C. Nicasio, S. Trofimenko and P. J. Perez, Organometallics, 2004, 23, 253–256 CrossRef CAS.
- K. Omura, T. Uchida, R. Irie and T. Katsuki, Chem. Commun., 2004, 2060–2061 RSC.
- G. De Faveri, G. Ilyashenko and M. Watkinson, Chem. Soc. Rev., 2011, 40, 1722–1760 RSC.
- Q. H. Teng, Y. Sun, Y. Yao, H. T. Tang, J. R. Li and Y. M. Pan, ChemElectroChem, 2019, 6, 3120–3124 CrossRef CAS.
- J. H. Qin, M. J. Luo, D. L. An and J. H. Li, Angew. Chem., 2021, 133, 1889–1896 CrossRef.
- W. Zhang and S. Lin, J. Am. Chem. Soc., 2020, 142, 20661–20670 CrossRef CAS PubMed.
- H. Wang, Y. F. Du, M. Y. Lin, K. Zhang and J. X. Lu, Chin. J. Chem., 2008, 26, 1745–1748 CrossRef CAS.
- B. Huang, Z. Sun and G. Sun, eScience, 2022, 2, 243–277 CrossRef.
- A. M. Sheta, M. A. Mashaly, S. B. Said, S. S. Elmorsy, A. V. Malkov and B. R. Buckley, Chem. Sci., 2020, 11, 9109–9114 RSC.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |