 Open Access Article
Open Access ArticleExtraction of bioactive compounds from beach-cast brown algae: a review on accelerated solvent extraction and subcritical water extraction
Yu Zhang *a,
Kelly Hawboldt
*a,
Kelly Hawboldt a and
Stephanie MacQuarrieb
a and
Stephanie MacQuarrieb
aDepartment of Process Engineering, Faculty of Engineering and Applied Science, Memorial University of Newfoundland, St. John's, NL A1B 3X5, Canada. E-mail: yzhang22@mun.ca
bDepartment of Chemistry, Cape Breton University, Sydney, NS B1P 6L2, Canada
First published on 24th June 2024
Abstract
Brown algae accumulation on beaches, or beach-cast, can lead to negative environmental impacts. However, beach-cast brown algae harvested from coastlines is a potential resource of bioactive compounds for use in food, biomaterial, cosmetic, and pharmaceutical industries. The extraction and subsequent separation and purification of bioactive compounds using conventional Solid–Liquid Extraction (SLE) requires improvement for sustainability and efficiency. Subcritical water processes are a potential “greener” approach in extraction without a decrease in process performance. This review outlines the bioactive compounds (alginate, fucoidan, laminarin, phenolic compounds, and fucoxanthin) in beach-cast brown algae and summarizes and compares conventional SLE and pressurized water methods: Accelerated Solvent Extraction (ASE) and Subcritical Water Extraction (SCWE). ASE is typically used in characterization/analysis, while SCWE is more appropriate for production. Extraction rate models and challenges related to scale-up in ASE and SCWE are also reviewed. ASE and SCWE can selectively extract bioactive compounds by modifying temperature/pressure and solvent combinations, and minimize extraction time, maximize yields and rates, and reduce chemical/solvent usage compared to SLE. However, kinetic modeling and scaling up of pressurized systems for brown algae valorization is still in its infancy. Future research is required to determine the green solvent combinations, develop batches into continuous processes, balance extraction conditions with product quantity and purity, and scale up for industrial-scale production.
Sustainability spotlightThe accumulation of beach-cast brown algae represents value loss and environmental challenges for ecosystems and communities, such as foul odors, potential harm to local organisms, and greenhouse gas emissions. However, employing green technologies to extract more value (alginate, fucoidan, laminarin, phenolics, and fucoxanthin) from less offers a sustainable solution for valorizing this “waste” biomass. These value-added compounds have various applications in food, biomaterials, cosmetics, and pharmaceuticals. This work reviews recent advancements in pressurized liquid processes, focusing on accelerated solvent extraction and subcritical water extraction for extracting bioactive compounds from small to large-scale applications and integrating them into the biorefinery concept. Aligned with UN SDG 7 for affordable and clean energy, it promotes renewable biomass development for a more sustainable future. |
1 Introduction
Marine bioeconomy innovations are underway in Newfoundland and Labrador, Canada, with a focus on sustainably utilizing raw and underused marine biomass resources to create value-added products. This innovative approach aims to advance a circular economy by extracting more from less while minimizing energy consumption and waste generation.1 One promising avenue is the sustainable utilization of beach-cast algae, typically considered waste but increasingly being recognized as a potential source of bioactive compounds (comparable to aquaculture/wild-harvested algae) such as polysaccharides, phenolic compounds, proteins, lipids, and pigments. These compounds provide health benefits, including antioxidant, anti-inflammatory, antitumor, and antibacterial properties, with applications in animal feed, human food, biomaterials, cosmetics, and pharmaceuticals.2–4 However, the full potential of beach-cast algae remains untapped, as they are often disposed of in landfills, leading to value loss, environmental concerns, and greenhouse gas emissions.5Global macroalgae production (aquaculture and wild harvesting) reached 38 million tonnes (wet weight) in 2022, marking a 4% increase from 2020 and approximately a 217% increase from 2000 (12 million tonnes). This growth was primarily driven by macroalgae aquaculture growth, particularly in Asia. China leads global macroalgae production, accounting for 60%, followed by Indonesia (25%), the Republic of Korea (5%), and the Philippines (4%).6 Marine macroalgae can be classified into three groups depending on pigmentation and chemical structures: red (Rhodophyta), brown (Ochrophyta), and green (Chlorophyta) algae. Among these, brown algae, with over 1500 known species, are the most consumed globally, representing approximately 66.5% of algae consumption.7,8 Brown algae are rich sources of bioactive components such as alginate, fucoidan, laminarin, phenolics, and fucoxanthin and contain compounds that act as nutrients, antioxidants, antivirals, and antimicrobials.9,10 In order to determine the “best” processes and products from beach-cast brown algae, the material must be characterized in terms of bioactive concentration and physicochemical properties.
Extraction is a crucial step for the separation and purification of bioactive compounds for both quantification/characterization and industrial production purposes. Conventional methods for extracting bioactive compounds from algae biomass involve multiple steps influenced by factors such as temperature, extraction time, solvent choice, liquid-to-solid ratio/flow rate, and pre-treatment (drying and grinding).11 Solid–liquid extraction (SLE) is the most common method, using liquid solvents such as methanol, chloroform, ethanol, acetone, and aqueous solution at ambient/elevated temperatures and atmospheric pressure. However, conventional SLE methods are often time-consuming and have safety/toxicity issues associated with large quantities of organic solvent usage.12
More recent developments in pressurized liquid extraction (PLE) aim to increase extraction efficiency, reduce extraction time, and minimize chemical/solvent usage to mitigate the negative impacts of toxic solvents on human health and the environment and potentially improve the overall sustainability of the process.13 Two promising technologies that employ pressure are accelerated solvent extraction (ASE) and subcritical water extraction (SCWE).8,14 ASE utilizes liquid solvents under elevated temperatures and very high pressure (40–200 °C and approximately 100 bar), which is particularly effective for lab-scale analysis and quantification. Elevated temperature and pressure enhance mass transfer rates, promote solvent penetration into solids and increase the solubility of target compounds, leading to faster extraction rates.13,15 SCWE is a specific case of PLE, using subcritical water (SCW) as the solvent, as with ASE. However, the pressure of the system is typically set by the temperature, resulting in SCWE pressures <100 bar at the same temperature as ASE. The lower operating pressure makes SCWE more suitable for industrial-scale recovery versus the analytical scale of ASE.16,17
Although extensive works have reviewed advanced PLE technologies, including ASE and SCWE of bioactive compounds from marine macroalgae,8,11,13,18–21 there is little study focused on beach-cast brown algae and ASE and SCWE processes at both small and large scales. Beach-cast algae will be weathered relative to the fresh counterpart, and therefore, process conditions using fresh algae may not be a good match for beach-cast.
In this context, this review will bridge the knowledge gap by highlighting beach-cast brown algae and their bioactive compound extraction using two “green” pressurized technologies (ASE and SCWE), focusing on quantification/characterization and industrial scaling-up. It will first provide an overview of biomass availability and potential bioactive compounds in beach-cast brown algae. This review will then focus on the extraction of these compounds using SLE, ASE, and SCWE. The comparisons of ASE and SCWE and their advantages and disadvantages are discussed. Studies on the economic and environmental sustainability of using these techniques for brown algae valorization will also outlined. Given the growing interest in ASE and SCWE as emerging alternatives to conventional SLE, understanding their extraction kinetics and mechanisms becomes crucial for predicting properties on a large scale. This review will summarize the scale-up and kinetic models of plant-based compound extraction using ASE and SCW to highlight the use of these techniques to obtain active compounds from beach-cast brown algae for a large-scale process.
2 Beach-cast brown algae
The accumulation of beach-cast algae has significantly increased in recent years, potentially due to climate change. The generation rate was estimated as 1 kg per square meter per year on a dry weight (dry wt) basis, with a turnover rate of around 2.6% per day.22 Therefore, this substantial accumulation of beach-cast algae plays an essential ecological role within marine ecosystems.1 Harb and Chow2 comprehensively reviewed beach-cast algae, emphasizing their ecological significance and potential environmental issues, health concerns, and financial losses. For example, extensive algae left on beaches leads to waste accumulation, unpleasant odors, greenhouse gas emissions from decomposition, and adverse effects on the tourism industry in coastal regions worldwide.Beach-cast brown algae can be harvested as a valuable resource for renewable materials and innovative products. Pardilhó et al.3 explored the feasibility of zero-waste biorefinery approaches on the valorization of algae waste into products, aligning with circular and blue economy principles. Applications of beach-cast brown algae include human food, animal feed, fertilizers, biochemical production, gelling agents, biofuels, and novel biomaterials.2,23,24 Brown beach-cast Laminaria hyperborea is commercially harvested in Scotland to produce soaps and glasses.25 In Ireland, alginate extracted from beach-cast brown algae Ascophyllum nodosum is used in human food, animal feed, and cosmetic industries.26 Bertagnolli et al.27 harvested beach-cast brown algae Sargassum filipendula from Brazilian beaches for alginate extraction. Canada has engaged in the commercial harvesting of brown algae by OrganicOcean, including Ascophyllum nodosum, Saccharina longicruris, and Fucus vesiculosus. Australia also reported commercial beach-cast brown algae harvesting for high-value alginate and low-value products such as fertilizer and animal feed.28 The global market for algae hydrocolloid alginate was valued at 345 million USD in 2015. Non-hydrocolloid polysaccharides (fucoidan and laminarin) have high market prices at 390 USD per kg and 52 USD per kg, respectively, due to their biological activities.29,30 The global market size by sales volume for fucoidan and laminarin is estimated at 17–18 t per year and 25–26 t per year, projected to grow at 3.8% and 8.7% compound annual growth rate, respectively.31 Thus, the high value and growing markets make brown algae valorization a promising business case.
The suitability of beach-cast brown algae for commercial applications depends on several factors, including biomass quality, species, target compound concentration, resource availability, and local market demands.3,4 Applications such as bioenergy, biofertilizers, and animal feed often do not require high-quality or homogenous quantities of brown algae. However, higher-value products demand high-quality feedstock. Due to the nutritional profiles and rich content of bioactive compounds in brown algae, the food, cosmetic, and pharmaceutical industries are typically the target market.3,32 Recent reviews suggest that beach-cast brown algae may have similar potential to fresh brown algae for downstream applications.2,33 Therefore, beach-cast brown algae could offer economic and ecological advantages, making them a sustainable biomass resource that improves environmental preservation and human health.
3 Potential bioactive compounds in beach-cast brown algae
3.1 Biomass availability and bioactive compounds concentration
Brown algae comprise a diverse group of over 1800 species classified into 20 classes. Among these, species belonging to the genera Ascophyllum, Sargassum, Fucus, Laminaria/Saccharina, and Undaria stand out for commercial value and bioactive potential.34 Ascophyllum nodosum is mainly cultivated/harvested from beaches. They are widely distributed on the rocky shores throughout the North Atlantic, including the northeastern coast of North America from New York to Newfoundland.35 Brown algae genera Fucus, Sargassum, Laminaria/Saccharina, and Undaria can be cultivated and wild-harvested.18 A comprehensive review by Pardilhó et al.3 summarized global beach-cast algae accumulations, detailing species, quantities, and current commercial applications.Brown algae are a source of nutrients (lipids, carbohydrates, proteins, minerals, and vitamins) and bioactive compounds (polysaccharides, phenolic compounds, and fucoxanthin) with various biological activities (Fig. 1). As shown in Table 1, brown algae genera Ascophyllum, Sargassum, and Fucus are rich in polysaccharides and phenolic compounds, accounting for up to 66–70 dry wt% and 12.7–14.0 dry wt% in gallic acid equivalents (GAE), respectively.18,34 Fucoxanthin is present in the highest concentrations in the Fucus and Undaria genera, reaching up to 4.36 and 5.41 mg per g dry wt, respectively.18 Bioactive compound concentrations vary among brown algae species and are influenced by environmental factors such as harvesting time, geographic habit, water temperature, light intensity, and nutrient levels.10,27
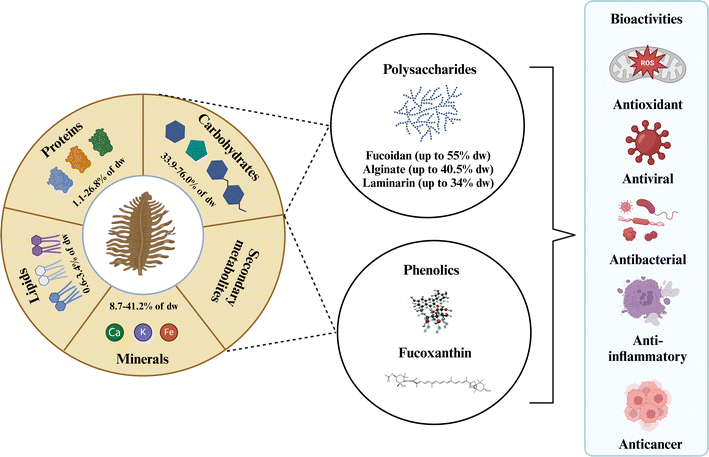 | ||
| Fig. 1 The composition of brown algae with their potential bioactive compounds. Created with https://www.biorender.com/. | ||
| Ascophyllum spp. | Sargassum spp. | Fucus spp. | Laminaria spp./Saccharina spp. | Undaria spp. | Ref. | |
|---|---|---|---|---|---|---|
| Polysaccharides (dry wt%) | 42–70 | 68 | 62–66 | 38–61 | 35–45 | 34 |
| Fucoidan (dry wt%) | 3.9–12 | 1–4.5 | 16–20 | 2–55% | 1.5–33% | 18 |
| Alginate (dry wt%) | 18.3–23.7 | 0.6–35 | 25 | 31.1–40.5 | Not applicable | 36 |
| Laminarin (dry wt%) | 1.2–10 | 0.3 | 84% of total sugars | 22–34 | 3 | 18 |
| Phenolics (dry wt% GAE) | 0.5–14 | 0.063–12.7 | 0.4–12.2 | 0.032–5.3 | 0.08–0.4 | 18 |
| Fucoxanthin (mg per g dry wt) | 0.172–1.78 | 0.0133–2.023 | 0.172–4.36 | 0.22–1.06 | 2.81–5.41 | 18 |
3.2 Polysaccharides
Beach-cast brown algae bioactive polysaccharides (alginate, fucoidan, and laminarin) have been extracted by SLE, ASE, and SCWE methods and are potentially used in functional foods, cosmetics, and medical industries.21,37 The chemical structures of these polysaccharides are depicted in Fig. 2.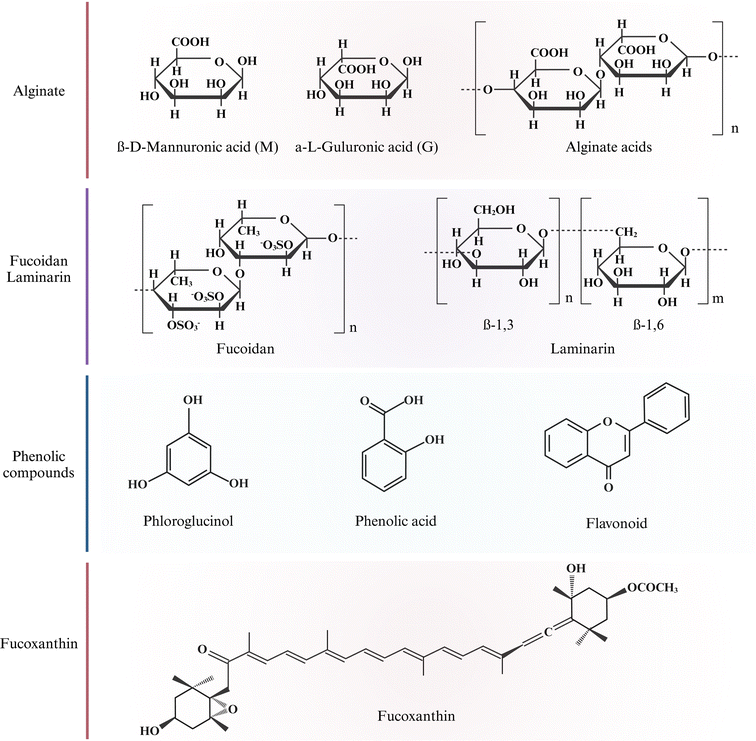 | ||
| Fig. 2 Chemical structure of bioactive compounds in brown algae. Created with https://www.biorender.com/. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio, and (v) drying the solid sodium alginate in an oven at 50–60 °C.
1 volume ratio, and (v) drying the solid sodium alginate in an oven at 50–60 °C.Alginate offers diverse properties and biological activities, including high viscosity, gelation, biodegradability, biocompatibility, and antibacterial and anticancer abilities. Alginate has applications in thickening/emulsifying agents, wound dressings, and functional food ingredients across industries.36
Several studies have attempted to integrate the extraction pathways of fucoidan, alginate, and laminarin, demonstrating the valorization potentials of brown algae species.45–48 Abraham et al.45 reported a method for co-extracting sodium alginate, fucoidan, and laminarin from beach-cast brown algae Durvillaea potatorum, incorporating an acid extraction step followed by an alkaline extraction. Similarly, Kostas et al.46 extracted alginate and fucoidan from Laminaria digitata. They also explored the potential of the solid residue as feedstock for bioethanol production and characterized the antioxidant and antimicrobial activities of the waste liquid stream. Yuan and Macquarrie47 developed a microwave-assisted step-by-step process for producing fucoidan, alginate, sugars, and biochar from Ascophyllum nodosum. Lorbeer et al.48 compared the sequential extraction of fucoidans and alginates from different beach-cast brown algae (Ecklonia radiata, Macrocystis pyrifera, Durvillaea potatorum, and Seirococcus axillaris), demonstrating process effectiveness when using different feedstocks. These studies are essential for maximizing the value of “waste” brown algae through zero-waste biorefinery approaches.
3.3 Phenolic compounds
Phenolic compounds are a group of secondary metabolites in plants consisting of one or multiple aromatic rings bonded to one or more hydroxyl groups. Brown algae are rich in phenolic compounds such as phlorotannins, phenolic acids, and flavonoids.49 Phlorotannins are derived from the polymerization of phloroglucinol (1,3,5-trihydroxybenzene) unit. They are highly hydrophilic across a wide range of MW range (126–650 kDa).50 Phenolic acids are a sub-class of phenolic compounds characterized by one carboxylic acid group.51 Flavonoids have 15 carbon skeletons and actively defend algae against oxidative damage.52 Fig. 2 shows phloroglucinol, phenolic acid, and flavonoid chemical structures. Phenolic compounds are widely used ingredients in healthy food due to their strong antioxidant abilities. Antioxidant compounds have a wide range of applications in food, supplements, and medicinal industries. The interaction between brown algae and environments will affect the distribution and concentration of phenolic compounds produced.83.4 Fucoxanthin
Fucoxanthin is a xanthophyll carotenoid abundant in brown algae and is responsible for its coloration.53 It has a unique chemical structure that contains nine conjugated double bonds, an allenic bond, epoxy, hydroxyl, carbonyl, and carboxyl groups in the molecule, as shown in Fig. 2. Fucoxanthin is a linear polymer with lower polarity, typically extracted from brown algae genera Undaria, Sargassum, and Laminaria using organic solvents.54 The concentration of fucoxanthin varies depending on species, geographical location, and harvesting season. For example, higher fucoxanthin levels are observed during winter when sunlight exposure and ocean temperatures are low. Fucoxanthin demonstrates diverse potential health benefits, including antioxidant, anti-inflammatory, anticancer, anti-obesity, and anti-diabetic effects, providing broad applications as a promising bioactive compound.554 Solid–liquid extraction
4.1 Extraction mechanisms
Conventional SLE, also referred to as leaching, is a widely used method for extracting bioactive compounds for characterization or production. It involves the transfer of solutes (one or more substitutes) from a solid or semisolid matrix to the liquid phase. The mechanism of SLE is made up of five steps:56 (i) wetting the sample matrix with liquid solvents; (ii) desorption of compounds from the solid matrix, including the breakdown of chemical bonds between solutes and solids; (iii) dissolution of compounds into the solvent; (iv) diffusion of solutes from the matrix to the external surface of the solids; (v) mass transfer of solutes from the solid surface into bulk solvents.Typically, the mass transfer of solute through the solid matrix is the rate-limiting step. Internal diffusion can be enhanced by reducing particle size and breaking down cell walls. External mass transfer can be improved by mixing/stirring in batch systems or increasing the flow rate in continuous-flow systems. The efficiency of solvent extraction (rate and yield) is a function of the target compound(s), liquid solvents, extraction time, temperature, liquid-to-solid ratio/flow rate, and particle size of the solid matrix. Among these factors, the liquid solvent is critical as it influences the solubility, selectivity, and sustainability.56 Organic solvents are commonly used for extracting phenolics, fucoxanthin, and antioxidants due to the medium-to-high polarity of phenolics and the low-to-medium polarity of fucoxanthin. Conversely, due to the water-soluble nature of polysaccharides, acid/alkaline water is a common solvent for recovering fucoidan, alginate, and laminarin from beach-cast brown algae.21 Table 2 summarizes recent studies on optimizing SLE with organic/aqueous solvents for components extraction from beach-cast brown algae worldwide.
| Country, source | Target bioactive compounds and biological activity | Extraction conditions | Brown algae species | Solvents: total extraction yield*; bioactive compound yield**1; bioactive compound content**2 | Ref. |
|---|---|---|---|---|---|
| a * total extraction yield on a dry wt basis (dry wt%); **1 bioactive compound yield in g of dry wt basis of algae; **2 bioactive compound content in g of extract; RT = room temperature; TPC = total phenolic compounds; PGE = phloroglucinol equivalents; GAE = gallic acid equivalents. | |||||
| Brazil, beach-cast | Phenolics polysaccharides antioxidant | Methanol: RT, 72 h, 30 mL g−1 | D. jolyana (ES) | Methanol: 4.49 *; 77.30 mg PGE per g **1 | 57 |
| Water: 30.04 *; 69.51 mg PGE per g **1 | |||||
| Water: 80 °C, 9 h, 30 mL g−1 | D. jolyana (PB) | Methanol: 2.66 *; 141.55 mg PGE per g **1 | |||
| Water: 29.67 *; 118.51 mg PGE per g **1 | |||||
| D. polypodioides | Methanol: 2.55 *; 58.59 mg PGE per g **1 | ||||
| Water: 9.51 *; 79.82 mg PGE per g **1 | |||||
| Z. tournefortii | Methanol: 4.12 *; 89.85 mg PGE per g **1 | ||||
| Water: 12.02 *; 98.42 mg PGE per g **1 | |||||
| Spain, coast | TPC antioxidant antibacterial | 50 °C, 24 h, 20 mL/0.6 g | U. pinnatifida | Ethanol: 38.80 *; 3.68 mg GAE per g **2 | 58 |
| Acetone: 3.40 *; 41.50 mg GAE per g **2 | |||||
| H. elongata | Ethanol: 27.00 *; 30.26 mg GAE per g **2 | ||||
| Acetone: 3.60 *; 162.22 mg GAE per g **2 | |||||
| B. bifurcata | Ethanol: 24.10 *; 11.39 mg GAE per g **2 | ||||
| Acetone: 10.80 *; 86.08 mg GAE per g **2 | |||||
| S. muticum | Ethanol: 17.90 *; 8.31 mg GAE per g **2 | ||||
| Ethyl acetate: 0.30 *; 41.63 mg GAE per g **2 | |||||
| L. ochroleuca | Ethanol: 19.20 *; 2.81 mg GAE per g **2 | ||||
| Ethyl acetate: 1.40 *; 32.46 mg GAE per g **2 | |||||
| S. latissima | Ethanol: 18.00 *; 2.44 mg GAE per g **2 | ||||
| Acetone: 2.90 *; 16.53 mg GAE per g **2 | |||||
| P. canaliculata | Ethanol: 15.60 *; 49.49 mg GAE per g **2 | ||||
| Acetone: 12.20 *; 87.32 mg GAE per g **2 | |||||
| F. spiralis | Ethanol: 14.60 *; 95.75 mg GAE per g **2 | ||||
| Acetone: 7.80 *; 184.22 mg GAE per g **2 | |||||
| A. nodosum | Ethanol: 18.20 *; 117.2 mg GAE per g **2 | ||||
| Ethyl acetate: 10.30 *; 211.83 mg GAE per g **2 | |||||
| Ireland, coast | TPC antioxidant | Organic solvent: RT, 24 h, 10 mL g−1 | L. digitata | Ethanol/water (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20): 35.20 *; 1.39 μg GAE per mg **2 20): 35.20 *; 1.39 μg GAE per mg **2 |
59 |
Methanol/water (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30): 36.50 *; 2.93 μg GAE per mg **2 30): 36.50 *; 2.93 μg GAE per mg **2 |
|||||
| Cold water: RT, 24 h, 20 mL g−1 | Cold water: 39.50 *; 2.24 μg GAE per mg **2 | ||||
| Hot water: 7.90 *; 5.06 μg GAE per mg **2 | |||||
| Hot water: 60 °C, 24 h, 20 mL g−1 | F. serratus | Ethanol/water (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20): 24.90 *; 75.96 μg GAE per mg **2 20): 24.90 *; 75.96 μg GAE per mg **2 |
|||
Methanol/water (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30): 26.30*; 80.70 μg GAE per mg **2 30): 26.30*; 80.70 μg GAE per mg **2 |
|||||
| Cold water: 35.90 *; 81.17 μg GAE per mg **2 | |||||
| Hot water: 6.20 *; 79.49 μg GAE per mg **2 | |||||
| Korea, coast | Fucoxanthin | RT, 20 h, 20 mL g−1 | S. horneri | Hexane: 1.42 *; 0.05 mg fucoxanthin per g **2 | 60 |
| Fatty acids | Ethanol: 1.36 *; 0.08 mg fucoxanthin per g **2 | ||||
| Antioxidant | Acetone/methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v): 1.29 *; 0.71 mg fucoxanthin per g **2 1, v/v): 1.29 *; 0.71 mg fucoxanthin per g **2 |
||||
| Antimicrobial | S. japonica | Hexane: 1.24 *; 0.16 mg fucoxanthin per g **2 | |||
| Antihypertensive | Ethanol: 1.22 *; 0.12 mg fucoxanthin per g **2 | ||||
Acetone/methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v): 1.19 *; 0.48 mg fucoxanthin per g **2 1, v/v): 1.19 *; 0.48 mg fucoxanthin per g **2 |
|||||
| China, coast | Polysaccharides | 84 °C, 4.3 h, 27 mL g−1 | A. nodosum | Water: 9.15 * | 61 |
| Antioxidant | |||||
| Immunostimulatory | |||||
| China, coast | Polysaccharides | 80 °C, 4 h for water/8 h for citric acid/NaOH, 50 mL g−1 | L. japonica | Water: 10.25 * | 62 |
| Antioxidant | Citric acid (pH 2): 11.23 * | ||||
| NaOH (pH 10): 44.63 * | |||||
4.2 SLE of bioactive compounds using organic solvents
Organic solvents are extensively used to extract bioactive compounds from brown algae harvested from beaches, coasts, and wild environments (Table 2). Brown algae contain diverse metabolites with varying concentrations and unique biological activities.10As shown in Table 2, extraction yields vary across algae species and solvent systems. Harb et al.57 analyzed the antioxidant activities and chemical composition of four beach-cast brown algae and nine green/red algae from Brazilian beaches. Results showed that extracts from beach-cast brown algae (Dictyopteris jolyana, Dictyopteris polypodioides, Zonaria tournefortii) exhibited the highest antioxidant activities, followed by eight red algae and one green algae. Similarly, Harb et al.63 found that extracts from four tested Brazilian brown algae wastes displayed the highest antioxidant activity, followed by two red and one green algae. Heffernan et al.59 also reported that beach-cast brown algae (Fucus serratus) had total phenolic content (TPC) and antioxidant activities thirty times higher than the other beach-cast red algae (Gracilaria gracilis) and green algae (Codium fragile). The above three studies observed a positive correlation between TPC and antioxidant activity.
Harb et al.57 evaluated four organic solvent systems, and methanol exhibited higher extraction yields compared to hexane, dichloromethane, and ethyl acetate. Methanol extracts were rich in phenolic compounds and sulfated polysaccharides, showing potential for antioxidant applications. However, this study only analyzed the highest-yield extracts (methanol), and the highest yield does not always translate to the highest quality (extract composition and functionality). Silva et al.58 compared five organic solvents (ethanol, acetone, ethyl acetate, chloroform, and hexane) for extraction yield, TPC, antioxidant capacity, and antibacterial abilities of nine brown algae. The highest extract yield was achieved when using ethanol as a solvent, while ethyl acetate/acetone extracts exhibited the highest TPC and antioxidant capacity. Heffernan et al.59 extracted phenolics and antioxidants from Brazilian beach-cast brown algae Laminaria digitata and Fucus serratus using 80 vol% ethanol and 70 vol% methanol as solvents. Slightly higher extract yields and TPC were observed with 70 vol% methanol, while ferric-reducing antioxidant power (FRAP) was higher in 80 vol% ethanol. Periaswamy Sivagnanam et al.60 investigated fucoxanthin extraction from Korean brown algae (Saccharina japonica and Sargassum horneri) collected from the coast. Three solvents (hexane, ethanol, and a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 vol% mixture of acetone/methanol) were compared in SLE at room temperature for 20 h. While the acetone/methanol mixture exhibited lower extraction yields compared to hexane and ethanol, it yielded higher fucoxanthin contents for both Saccharina japonica and Saccharina horneri. Specifically, the fucoxanthin content in the acetone/methanol mixture was three times the value of the hexane extract (0.48 mg g−1 vs. 0.16 mg g−1 of crude extract) and four times the ethanol extract (0.12 mg g−1 of crude extract) for Saccharina japonica. This difference was more pronounced in Saccharina horneri, where hexane and ethanol extracts were 10–12% of the acetone/methanol fucoxanthin. This study also noted crude extracts were rich in phenolics, fatty acids, and fucoxanthin exhibiting antioxidant, antimicrobial, and antihypertension effects.
50 vol% mixture of acetone/methanol) were compared in SLE at room temperature for 20 h. While the acetone/methanol mixture exhibited lower extraction yields compared to hexane and ethanol, it yielded higher fucoxanthin contents for both Saccharina japonica and Saccharina horneri. Specifically, the fucoxanthin content in the acetone/methanol mixture was three times the value of the hexane extract (0.48 mg g−1 vs. 0.16 mg g−1 of crude extract) and four times the ethanol extract (0.12 mg g−1 of crude extract) for Saccharina japonica. This difference was more pronounced in Saccharina horneri, where hexane and ethanol extracts were 10–12% of the acetone/methanol fucoxanthin. This study also noted crude extracts were rich in phenolics, fatty acids, and fucoxanthin exhibiting antioxidant, antimicrobial, and antihypertension effects.
4.3 SLE of bioactive compounds using aqueous solvents
Water-based solvents are potentially greener options compared to organic solvents. As shown in Table 2, water has been widely used in bioactive extraction from beach-cast brown algae, mainly for polysaccharides. Aqueous extracts from four Brazil beach-cast brown algae showed 3–13 folds higher extraction yields compared to methanolic extracts. These aqueous extracts also exhibited higher ABTS+ scavenging and metal chelating activities for all four studied beach-cast brown algae compared to methanolic extracts.57 Similarly, another study on beach-cast algae (Fucus serratus) demonstrated that SLE using water at room temperature had higher extraction yield (35.90 dry wt%) and TPC (81.17 μg GAE per g extract) compared to organic solvents ethanol (yield of 24.9 dry wt% and 75.96 μg GAE per g extract) and methanol (yield of 26.3 dry wt% and TPC of 80.70 μg GAE per g extract).59Polysaccharides are highly soluble in water but insoluble in organic solvents such as ethanol and acetone. Therefore, the typical process is water extraction followed by ethanol precipitation. Chen et al.61 optimized water extraction of polysaccharides from Ascophyllum nodosum using the Box–Behnken design (BBD). The “best” crude polysaccharide yield of 9.15 ± 0.23 dry wt% was obtained at an extraction time of 4.3 h, a temperature of 84 °C, and a water-to-solid ratio of 27 mL g−1. Sun et al.62 studied the impact of acidic, water, and alkaline effects on polysaccharide yields, structural features, and antioxidant activities from brown algae Laminaria japonica. Alkaline extraction (NaOH, pH 10) achieved the highest polysaccharide yield (44.63 dry wt%), surpassing pH 2 citric acid (11.23 dry wt%) and water (10.25 dry wt%) extraction. SLE under acidic and alkaline conditions showed extracts with irregular and rough particles and stronger antioxidant activities, including free radical scavenging, Fe2+ chelating capacity, and lipid peroxidation inhibition. Peasura et al.64 observed similar trends in green algae Ulva intestinalis, where alkaline extraction yielded higher sulfated polysaccharide yields (14 dry wt%) compared to distilled water (10 dry wt%) and 0.1 M HCl (12 dry wt%) at the same conditions (80 °C, 6 h, and a liquid-to-solid ratio of 20 mL g−1). However, polysaccharides extracted at acidic conditions exhibited better antioxidant activity than those from water and alkali. These results suggest that adding acid/alkali can help break down chemical bonds between cellulose in brown algae cell walls, facilitating the release of polysaccharides into the solvent. However, caution is needed in conventional acid/base treatments due to the potential risk of breaking covalent bonds in the targeted solute of interest.52
5 Accelerated solvent extraction
Bioactive compound extraction using liquid solvents is common in analytical laboratories due to its simple setup. However, the process is often time-consuming (usually 4–72 h) and requires large volumes of organic solvents (approximately 10–50 mL per g biomass). Concerns about organic solvent usage, associated human exposure, and increased purchase and disposal costs have highlighted the need for more efficient extraction methods. In response to these concerns, ASE was introduced. ASE is a higher-pressure variation (40–200 °C, approximately 100 bar) of conventional SLE where the same solvents can be used. However, the increased pressure and temperature produce high-diffusion liquids. The resulting faster extraction rates lead to reduced extraction time, solvent savings, and increased yields. The automatic ASE system does not typically require an extra pre-treatment step (separation or concentration), making it ideal for analytical analysis (quantification/characterization).13 This section discusses recent developments in ASE for extracting bioactive compounds from beach-cast brown algae.5.1 Principles and mechanisms
The ASE technique was first introduced in 1996 by Richter et al.65 The Dionex Corporation (Thermo Fisher Scientific, USA) subsequently commercialized extraction units, including ASE 150, 200, and 350 systems, which have been widely used by many laboratories worldwide. As noted above, ASE operates at 40–200 °C and approximately 100 bar. The extraction is modeled as a four-stage process:14 (i) solvent penetration into solids, (ii) solute(s) desorption/dissolution, (iii) internal diffusion of solute(s), and (iv) external mass transfer of solute(s) into the bulk flowing fluid (Fig. 3). Elevated temperature increases solvent diffusivity by reducing solvent viscosity and surface tension, accelerating the extraction rates. Pressure maintains the solvent in a liquid state at temperatures above its boiling point, facilitates a more rapid extraction cell filling, and more effective solvent penetration. Furthermore, the pressurized solvent enhances the solubility of targets, resulting in increased extraction rates and yields. The degree of enhancement is a function of not only pressure and temperature but also the solvent(s) of choice. For instance, when the solvent is water in ASE conditions, water is subcritical, and the process is low-polarity water extraction. However, high temperatures can degrade thermolabile compounds such as pigments and alginate.66 Therefore, optimization of extraction conditions, especially temperature, is vital to maximize the quality and quantity of yield(s).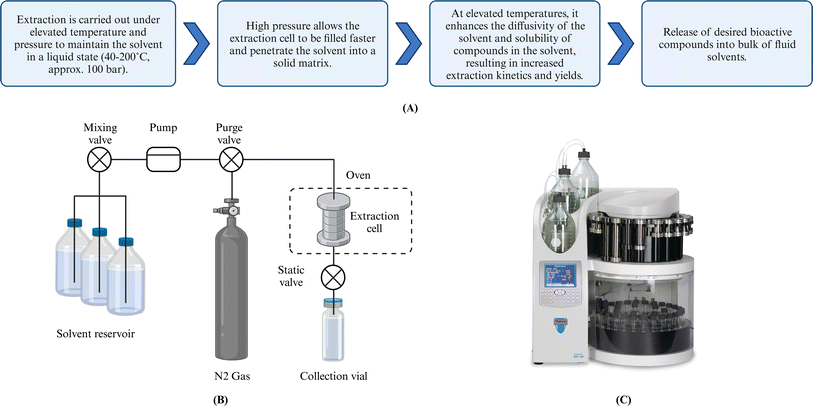 | ||
| Fig. 3 (A) Mechanism of ASE technique. (B) Schematic of the ASE Process. (C) Commercial automated accelerated solvent extractor. Created with https://www.biorender.com/. | ||
ASE has three operation modes: static, dynamic, and semi-dynamic.14 Most ASE studies involving bioactive compounds from brown algae focus on static mode (Table 3). In this mode, pressurized solvent reacts with the solid matrices for a fixed time at constant pressure and temperature before collection. Although the static mode uses less solvent than other modes, the extraction efficiency is limited once equilibrium concentration in the solute is achieved. Thermal degradation and undesirable chemical reactions can occur after equilibrium. Dynamic ASE employs a continuous supply of fresh pressurized liquid, avoiding equilibrium but potentially requiring more solvent and reducing contact time for desorption and diffusion. Semi-dynamic extraction, also known as the multiple rinse cycle mode, combines static and dynamic features. It begins with a static period ensuring desorption/internal diffusion, followed by dynamic flow by introducing fresh solvent to shift equilibrium.
| Country, source, species | Extractor | Extraction parameters | Bioactive compounds | Yields | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| Solvents (v/v) | T (°C) | P (bar) | t (min) | L/S (mL g−1) | |||||
| a T = temperature (°C), P = pressure (bar), t = total extraction time (min), including ramp-up time and static time, L/S = liquid-to-solid ratio (mL g−1), GAE = gallic acid equivalents, PGE = phloroglucinol equivalents. | |||||||||
| Denmark, beach, F. vesiculosus | ASE 350 | Water | 190 | 103 | 14 | 10 | Crude extract | 25.99 dry wt% | 67 |
| 140 | 103 | 12 | 10 | Alginate | 2.08 dry wt% | ||||
| 160 | 103 | 13 | 10 | Fucoidan | 12.52 dry wt% | ||||
| 200 | 103 | 14 | 10 | Phenolics | 51.04 mg GAE per g extract | ||||
| 150 | 103 | 12 | 10 | Phlorotannin | 2.24 mg per g extract | ||||
| Croatia, coast, F. virsoides | ASE 350 | 0.1M H2SO4 | 140 | 103 | 37 | 22 | Polysaccharides | 24.22 dry wt% | 68 |
| Spain, H. elongata | ASE 200 | Hexane | 50 | 103 | 25 | 11 | Crude extract | 3.41 dry wt% | 69 |
| 100 | 103 | 25 | 11 | Crude extract | 3.50 dry wt% | ||||
| 150 | 103 | 27 | 11 | Crude extract | 4.72 dry wt% | ||||
| 200 | 103 | 29 | 11 | Crude extract | 7.59 dry wt% | ||||
| Ethanol | 50 | 103 | 25 | 11 | Crude extract | 8.29 dry wt% | |||
| 100 | 103 | 25 | 11 | Crude extract | 10.56 dry wt% | ||||
| 150 | 103 | 27 | 11 | Crude extract | 19.23 dry wt% | ||||
| 200 | 103 | 29 | 11 | Crude extract | 36.91 dry wt% | ||||
| Water | 50 | 103 | 25 | 11 | Crude extract | 9.51 dry wt% | |||
| 100 | 103 | 25 | 11 | Crude extract | 15.08 dry wt% | ||||
| 150 | 103 | 27 | 11 | Crude extract | 46.43 dry wt% | ||||
| 200 | 103 | 29 | 11 | Crude extract | 51.56 dry wt% | ||||
| Spain, H. elongata | ASE 200 | Hexane | 100 | 103 | 25 | 13.8 | Crude extract | — | 70 |
| Ethanol | 100 | 103 | 25 | 7.3 | Crude extract | — | |||
| Water | 100 | 103 | 25 | 11 | Crude extract | — | |||
| Denmark, beach, F. vesiculosus | ASE 350 | Ethanol/water (58.65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41.35) 41.35) |
137.18 | 103 | 10 | 10 | Crude extract | 31.16 dry wt% | 71 |
| 137.18 | 103 | 10 | 10 | Phenolics | 3.69% GAE | ||||
| Ireland, coast, F. serratus | ASE 200 | Water | 120 | 103 | 25 | 8.8 | Crude extract | 33.40 dry wt% | 59 |
| 120 | 103 | 25 | 8.8 | Phenolics | 21.32 μg GAE per g extract | ||||
Ethanol/water (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) 20) |
100 | 69 | 25 | 8.8 | Crude extract | 31.70 dry wt% | |||
| 100 | 69 | 25 | 8.8 | Phenolics | 56.68 μg GAE per g extract | ||||
Methanol/water (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) 30) |
90 | 69 | 25 | 8.8 | Crude extract | 29.20 dry wt% | |||
| 90 | 69 | 25 | 8.8 | Phenolics | 61.11 μg GAE per g extract | ||||
| Ireland, coast, L. digitata | ASE 200 | Water | 120 | 103 | 25 | 8.8 | Crude extract | 29.80 dry wt% | |
| 120 | 103 | 25 | 8.8 | Phenolics | 1.37 μg GAE per g extract | ||||
Ethanol/water (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) 20) |
100 | 69 | 25 | 8.8 | Crude extract | 24.80 dry wt% | |||
| 100 | 69 | 25 | 8.8 | Phenolics | 2.20 μg GAE per g extract | ||||
Methanol/water (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) 30) |
90 | 69 | 25 | 8.8 | Crude extract | 28.20 dry wt% | |||
| France, wild, S. muticum | ASE 200 | Ethanol/water (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) 5) |
90 | 69 | 25 | 8.8 | Phenolics | 2.18 μg GAE per g extract | 72 |
| 160 | 103 | 26 | 5.5 | Crude extract | 21.90 dry wt% | ||||
| 160 | 103 | 26 | 5.5 | Phenolics | 94 mg GAE per g extract | ||||
| 160 | 103 | 26 | 5.5 | Phlorotannins | 5.02 mg PGE per g extract | ||||
| Korea, coast, E. bicyclis | ASE | Ethanol/water (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) 10) |
110 | 103 | 11 | 5.5 | Fucoxanthin | 0.42 mg g−1 | 73 |
| Spain, coast, L. ochroleuca | ASE 350 | Hexane | 120 | 103 | 15 | 10 | Crude extract | 9.02 dry wt% | 74 |
| 160 | 103 | 18 | 10 | Crude extract | 7.43 dry wt% | ||||
| Ethyl acetate | 120 | 103 | 15 | 10 | Crude extract | 7.42 dry wt% | |||
| 160 | 103 | 18 | 10 | Crude extract | 5.29 dry wt% | ||||
| Ethanol | 120 | 103 | 15 | 10 | Crude extract | 15.54 dry wt% | |||
| 160 | 103 | 18 | 10 | Crude extract | 11.55 dry wt% | ||||
Ethanol/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
120 | 103 | 15 | 10 | Crude extract | 47.16 dry wt% | |||
| 160 | 103 | 18 | 10 | Crude extract | 51.91 dry wt% | ||||
5.2 ASE of bioactive compounds from beach-cast brown algae
The studies outlined below include bioactive compound extraction from dried and ground brown algae powder and ASE combined with separation (e.g., precipitation and purification) to extract phenolics and polysaccharides. Antioxidant activity is one of the most extensively studied biological properties. As shown in Table 3, overall crude extract yields vary with species and extraction parameters. The effects of solvents and temperatures are the most extensively studied. Getachew et al.67 investigated ASE using water for bioactive compound extraction from beach-cast brown algae Fucus vesiculosus. They found the highest yield (25.99 dry wt%), TPC (51.04 mg GAE/g extract), and antioxidant activities were achieved between 190-200 °C. However, the highest alginate yield (2.08 dry wt%) and fucoidan yield (12.52 dry wt%) were observed at 140 °C and 160 °C, respectively. The decrease in polysaccharide yield at higher temperatures may be due to thermal degradation or Maillard reactions, leading to polysaccharide breakdown into small organic acids. Dobrinčić et al.68 reported a comparable temperature range for sulfated polysaccharide extraction from brown algae Fucus virsoides harvested from the southwest coast of the Novigrad Sea, Croatia. The highest polysaccharide yield (24.22 dry wt%) was achieved at 140 °C, with a liquid-to-solid ratio of 22 mL g−1, 103 bar pressure, over 37 min, using 0.1 M H2SO4 as a solvent.Plaza et al.69 studied ASE as an analytical method to characterize bioactive compounds for antioxidant and antimicrobial activities from brown algae Himanthalia elongata. Different solvents (hexane, ethanol, and water) and temperatures (50, 100, 150, and 200 °C) were evaluated for impact on crude extract yield, chemical composition, and biological activities. As with previous work, higher temperatures resulted in increased extract yield. Solvents with higher polarity led to greater extraction yield, indicating major compounds in Himanthalia elongata tend to be medium to highly polar. In another antiviral study, Santoyo et al.70 focused on Himanthalia elongata and found that ethanol extracts were able to better inhibit virus infection, approximately 90% at a concentration of 75 μg mL−1, compared to water and hexane extracts, which reduced virus infectivity to 78% and 70%, respectively. Subsequent characterization of extracts revealed that the antiviral activity of water extracts correlated with polysaccharides, while ethanol and hexane extracts were associated with fucosterol.
Several studies have explored solvent mixtures to balance yield and quality/biological activity. Studies of ethanol/water mixtures dominate, as ethanol is generally recognized as safe (GRAS) and suitable for use in the food industry. Heffernan et al.59 compared the impact of water, 80 vol% ethanol, and 70 vol% methanol on the extraction yield, TPC, and antioxidant activity from brown algae Fucus serratus and Laminaria digitata harvested from coastal areas. The highest yield (33.40 dry wt%) was achieved for Fucus serratus using water as a solvent, surpassing aqueous ethanol (31.70 dry wt%) and aqueous methanol (29.20 dry wt%). However, ethanol/water and methanol/water extracts showed higher TPC and antioxidant activities compared to water extracts, demonstrating the role operating conditions play in quantity and quality. Sumampouw et al.71 optimized ASE conditions to achieve high extract yield, TPC, and strong antioxidant properties from beach-cast brown algae Fucus vesiculosu. Optimal conditions (137.18 °C, 58.65 vol% ethanol in water for 4.68 min) produced a yield of 31.16 dry wt%, phenolic yield of 3.69 g GAE/100 g dry wt, and effective concentrations for 50% inhibition (EC50) of DPPH radical (92.60 μg mL−1), ABTS radical (2.35 mg mL−1), and metal chelating (1.10 mg mL−1). In another study, Sánchez-Camargo et al.72 compared ASE with enzyme-assisted extraction and alkaline hydrolysis, with results showing that ASE alone produced the highest yields and antioxidant activity. The maximum yield (21.90 dry wt%), TPC (94.00 mg GAE/g extract), phlorotannins content (5.02 mg per g extract), and antioxidant activity (1.28 mmol Trolox equivalents per g extract) were obtained at 160 °C and 95 vol% ethanol conditions.
ASE has been studied to extract fucoxanthin and fatty acids. Shang et al.73 optimized the extraction conditions for fucoxanthin from brown algae using a design of experiment approach. Solvent and temperature were the most significant factors, achieving a maximum fucoxanthin yield of 0.42 mg g−1 at 110 °C with 90 vol% ethanol. Otero et al.74 assessed fatty acids and TPC extracted from brown algae Laminaria ochroleuca using ASE. Four solvent systems (hexane, ethyl acetate, ethanol, and ethanol/water 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) were compared, with the ethanol/water mixture giving the highest yield (51.91 dry wt%), TPC (173.65 mg GAE per g extract), and antioxidant activity at 160 °C and 18 min extraction time. While ASE has shown excellent utility in characterizing and high yields of bioactive compounds in algae, running a system at over 100 bar can be challenging as one scales up. As such, SCWE, which runs at lower pressures, maybe a better option for production.
1, v/v) were compared, with the ethanol/water mixture giving the highest yield (51.91 dry wt%), TPC (173.65 mg GAE per g extract), and antioxidant activity at 160 °C and 18 min extraction time. While ASE has shown excellent utility in characterizing and high yields of bioactive compounds in algae, running a system at over 100 bar can be challenging as one scales up. As such, SCWE, which runs at lower pressures, maybe a better option for production.
6 Subcritical water extraction
Subcritical water (SCW) is defined as water at pressures and temperatures to maintain the water in the liquid phase even though above the normal boiling point of water. This translates to pressures between 10-221 bar and temperatures between 100–374 °C.75 Subcritical water extraction (SCWE) utilizes SCW as a green solvent, also known as pressurized hot water extraction (PHWE) or superheated water extraction. When water is used as the sole solvent in an ASE system under operational conditions of 100–200 °C and approximately 100 bar, ASE can also be considered SCWE in this specific context. Both ASE and SCWE offer faster extraction rates, reduced solvent usage, and higher yields compared to conventional SLE methods.13 However, unlike ASE, SCWE is more readily scalable for industrial use due to its ability to operate at lower pressures (water vapor pressure).526.1 Principles and mechanisms
The SCWE of bioactive compounds from solid/semisolid samples can generally be described as the same five stages as SLE,56 as outlined in Section 4.1. Operation parameters such as temperature, pressure, extraction time/flow rate, and particle size influence the five extraction stages. Fig. 4 shows the P–T diagram for water and the subcritical region and depicts a typical lab-scale pressurized reactor.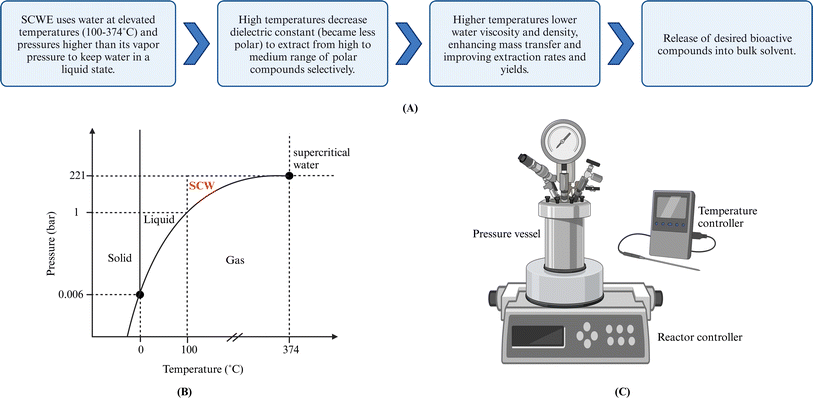 | ||
| Fig. 4 (A) Mechanism of SCWE technique. (B) Water phase diagram. (C) Lab-scale SCW Batch reactor. Created with https://www.biorender.com/. | ||
As noted in ASE, the elevated temperature reduces water viscosity and surface tension while enhancing diffusivity, allowing deep solvent penetration into the solid matrix and improving mass transfer. The temperature increase also reduced intermolecular forces between solutes and solids, resulting in faster desorption of solutes from the matrix.17 However, the solubility effect can impact both target and nontarget compounds, resulting in a less selective process. In addition, other chemical reactions and thermal degradation may occur under high temperature and pressure conditions.52
| Country, source, species | Batch reactor | Target compounds | Solvents | P (bar) | T (°C) | Ramp-up time (min) | Static time (min) | L/S (mL g−1) | Yields | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| a T = temperature (°C), P = pressure (bar), Ramp-up time is the extraction time needed to reach the specified extraction temperature; Static time represents the isothermal extraction time after reaching the specified extraction temperature; Cooling time was not given by most studies; NG = not given; L/S ratio = liquid-to-solid ratio; GAE = gallic acid equivalents, PGE = phloroglucinol equivalents. | ||||||||||
| New Zealand, wild, U. pinnatifida | 1 L | Phenolics | Water | 30 | 210 | 15–20 | 15–20 | 100 | 29.90 mg GAE per g | 79 |
| Phlorotannin | Water | 30 | 210 | 15–20 | 20 | 100 | 0.99 mg PGE per g | |||
| Fucoidan | Water | 30 | 120 | 15–20 | 5 | 50 | 4.60 dry wt% | |||
| Iran, beach, N. zanardinii | 500 mL | Fucoidan | Water | 7.5 | 150 | 5 | 29 | 21 | 25.98 dry wt% | 80 |
| Iran, coast, N. zanardinii | 500 mL | Fucoidan | Water | 7.5 | 150 | 5 | 20 | 30 | 13.15 dry wt% | 81 |
| South Africa, coast, E. maxima | 1 L | Crude extract | Water | 40 | 180 | NG | 23.75 | 30 | 76.02 dry wt% | 82 |
| Phenolics | Water | 40 | 180 | NG | 23.75 | 30 | 41.20 mg GAE per g extract | |||
| Alginate | Water | 40 | 120 | NG | 5 | 30 | 15.65 dry wt% | |||
| Spain, algamar, H. elongata | 3.7 L | Crude extract | Water | 10.03 | 180 | NG | 0 | 30 | 70.70 dry wt% | 77 |
| Phenolics | Water | 23.18 | 220 | NG | 0 | 30 | 45.50 mg GAE per g extract | |||
| Fucoidan | Water | 6.2 | 160 | NG | 0 | 30 | 9.05 dry wt% | |||
| Carbohydrate | Water | 6.2 | 160 | NG | 0 | 30 | 38.88 dry wt% | |||
| Spain, algamar, L. ochroleuca | 3.7 L | Crude extract | Water | 7.5 | 220 | NG | 0 | 30 | 80.00 dry wt% | 78 |
| Phlorotannin | Water | 7.5 | 220 | NG | 0 | 30 | 3.20 g/100 g extract | |||
| Proteins | Water | 7.5 | 220 | NG | 0 | 30 | 18.00 g/100 g extract | |||
| Fucoidan | Water | 7.5 | 160 | NG | 0 | 30 | 41.38 g/100 g extract | |||
| Alginate | Water | 7.5 | 160 | NG | 0 | 30 | 14.94 g/100 g extract | |||
| Korea, S. japonica | 200 mL | Alginate | Water | 5 | 80 | 10 | 5 | 16.58 | 8.21 dry wt% | 40 |
| Alginate | 0.1% NaOH | 5 | 80 | 10 | 5 | 16.58 | 10.31 dry wt% | |||
| Alginate | 0.1% formic acid | 5 | 80 | 10 | 5 | 16.58 | 8.22 dry wt% | |||
| Alginate | 70% ethanol | 5 | 80 | 10 | 5 | 16.58 | 4.10 dry wt% | |||
| Alginate | 50% ethanol | 5 | 80 | 10 | 5 | 16.58 | 1.75 dry wt% | |||
| Alginate | 25% ethanol | 5 | 80 | 10 | 5 | 16.58 | 6.00 dry wt% | |||
| Fucoidan | Water | 50 | 140 | 15 | 5 | 16.58 | 6.52 dry wt% | |||
| Fucoidan | 0.1% NaOH | 50 | 140 | 15 | 5 | 16.58 | 8.23 dry wt% | |||
| Fucoidan | 0.1% formic acid | 50 | 140 | 15 | 5 | 16.58 | 7.00 dry wt% | |||
| Fucoidan | 70% ethanol | 50 | 140 | 15 | 5 | 16.58 | 0.50 dry wt% | |||
| Fucoidan | 50% ethanol | 25 | 110 | 10 | 5 | 16.58 | 1.95 dry wt% | |||
| Fucoidan | 25% ethanol | 25 | 110 | 10 | 5 | 16.58 | 2.90 dry wt% | |||
| Korea, S. japonica | 200 mL | Fucoidan | 0.1% NaOH | 80 | 127.01 | 12 | 11.98 | 25 | 13.56 dry wt% | 83 |
| Korea, S. japonica | 200 mL | Phenolics | Ionic liquids (0.25 M) | 50 | 175 | 25 | 5 | 32 | 58.91 mg PGE per g | 84 |
| Phenolics | Water | 50 | 175 | 25 | 5 | 32 | 39.27 mg PGE per g | |||
| Korea, S. japonica | 200 mL | Fucoidan | 30% DESs | 19.85 | 150 | 25 | 0 | 36.81 | 14.93 dry wt% | 39 |
| Alginate | 30% DESs | 19.85 | 150 | 25 | 0 | 36.81 | 28.12 dry wt% | |||
| Spain, beach-cast, D. dichotoma | 15 L | Glucose | Water | 5.12 | 150 | NG | 30 | 8 | 2.29 g L−1 | |
| Xylose | Water | 5.12 | 150 | NG | 30 | 8 | 3.11 g L−1 | 5 | ||
| Arabinose | Water | 5.12 | 150 | NG | 30 | 8 | 1.27 g L−1 | |||
As with ASE, operating in dynamic mode can increase extraction yield, staying below equilibrium concentrations and minimizing the risk of degradation or chemical reactions.52 The flow rate optimization is a function of a number of factors, including whether the extraction rate is external mass transfer controlled or internal mass transfer controlled.86 If external mass transfer dominates, then higher flow rates induce more turbulence and better mass transfer. However, this may result in diluted extracts (due to contact time). Internal mass transfer control is a function of diffusion rate, which is less sensitive to flow. It should be noted internal mass transfer resistances can be minimized by optimizing particle size.
6.2 SCWE of bioactive compounds from beach-cast brown algae
The bulk of SCWE work has focused on the recovery or production (versus characterization as in ASE) of bioactive compounds from brown algae, using a laboratory batch scale reactor from 200 mL to 3.7 L (Table 4). Crude extract yield varies considerably depending on the species and SCW conditions. Korean coastal-harvested brown algae Undaria pinnatifida showed a higher extract yield (62.37 dry wt%) compared to Laminaria japonica (35.53 dry wt%) or Hizikia fusiforme (18.55 dry wt%) under the same SCW conditions (210 °C, 30 bar, water-to-solid ratio of 20 mL g−1, and non-isothermal processing).87 Higher extract yields were reported from brown algae Himanthalia elongata (70.70 dry wt%)77 at 180 °C and Laminaria ochroleuca (80.0 dry wt%)78 at 220 °C using non-isothermal processing. A similar high yield (76.02 dry wt%) was produced in the South African brown algae Ecklonia maxima at 180 °C, 40 bar, water-to-solid ratio of 30 mL g−1 for 23.75 min isothermal static time.82 These crude extracts typically contain different compounds with medium to high polarity, such as antioxidants, phenolics, phlorotannin, fucoidan, and alginate. More selective extraction requires more precision in operating conditions and solvents.Gan and Baroutian79 reported the highest yields of phenolics (29.90 mg GAE per g dry wt) and phlorotannin (0.99 mg phloroglucinol equivalents (PGE) per g dry wt) at 210 °C and 15 or 20 min static time. These results were higher than conventional hot water extraction, where the phenolic yield was 1.27 mg GAE per g dry wt and the phlorotannin yield was 0.69 mg PGE per g dry wt. In a study of brown algae Laminaria ochroleuca, the highest phlorotannin content (3.20 g/100 g extract) in the extract was reported at 220 °C.78 Na et al.87 obtained the highest phenolic yields for Undaria pinnatifida (14.30 mg per g dry wt), Laminaria japonica (10.90 mg per g dry wt), and Hizikia fusiforme (18.10 mg per g dry wt) at 210 °C. Bordoloi and Goosen82 found the highest TPC (41.20 mg GAE per g extract) at 180 °C and 23.75 min static time. Cernadas et al.77 observed an increase in the TPC from 4.10 mg GAE per g extract at 120 °C to a maximum of 45.50 mg GAE per g extract at 220 °C. The above five studies have noted that the optimal temperature range for antioxidant activities aligns with the optimal TPC and phlorotannin content. Jacobsen et al.18 also reviewed a positive correlation between phenolics/phlorotannins and their antioxidant potential.
Whereas overall yields, TPC, phlorotannin, and antioxidant activity were favored at elevated SCW temperatures (>180 °C), polysaccharide yields were highest at lower temperature ranges (<160 °C). Optimal fucoidan yield (4.60 dry wt%) was obtained at lower temperatures and shorter isothermal static times (120 °C and 5 min) compared to TPC (210 °C and 15 min).79 Bordoloi and Goosen82 obtained the maximum alginate yield of 15.65 dry wt% at 120 °C with a 5 min static time. Slightly higher temperatures were also reported for the recovery of fucoidan (13.15 dry wt%)81 at 150 °C and alginate (14.94 dry wt%)78 at 160 °C. The highest fucoidan yield (25.98 dry wt%) from beach-harvested brown algae Nizamuddinia zanardinii was obtained at 150 °C, with a water-to-solid ratio of 21 mL g−1 and 29 min static time. This value was much higher at a much shorter residence time than the yield via conventional SLE (5.20 dry wt%) at 65 °C, with a water-to-solid ratio of 20 mL g−1 over 6 h.80 In another study, Alboofetileh et al.81 compared SCWE with other extraction methods. Results showed SCWE had the highest fucoidan yield (13.15 dry wt%) compared to enzyme-assisted extraction (4.28–5.58 dry wt%), ultrasound-assisted extraction (3.60 dry wt%), microwave-assisted extraction (6.17 dry wt%), enzyme-ultrasound-assisted extraction (7.87 dry wt%), and microwave-ultrasound-assisted extraction (5.53 dry wt%).
Adding organic and inorganic solvents may enhance the solubility of target compounds in SCW and affect the sample matrix structure.52 Saravana et al.40 investigated the effect of various solvents (water, 0.1% NaOH, 0.1% formic acid, 25–75% aqueous ethanol) on SCWE of fucoidan from Saccharina japonica. The best combination of crude fucoidan (8.23 dry wt%) and alginate (10.31 dry wt%) yields were obtained at 140 °C and 50 bar when using 0.1% NaOH, compared to pure water, 0.1% formic acid, and 25–75% aqueous ethanol. Alkaline water (0.1% NaOH) might induce cellulose swelling, which disrupts the hydrogen bonds within hemicellulose and cellulose in the cell wall structure, facilitating the release of polysaccharides. In subsequent work, Saravana et al.83 optimized fucoidan SCWE with 0.1% NaOH. The studied parameters included temperature (100–180 °C), pressure (20–80 bar), solvent/solid ratio (11–25 mL g−1), agitation speed (100–300 rpm), and static time (5–15 min). The optimal fucoidan yield (13.56 dry wt%) was at 127 °C, 80 bar, 25 mL g−1, 300 rpm, and 11.98 min.
Dinh et al.84 studied the combination of SCW with ionic liquids (ILs) in the extraction of phenolics from brown algae Saccharina japonica. SCW was compared to SCW+1-butyl-3-methylimidazolium tetrafluoroborate; concentrations of IL at 0.25 M led to an increased phenolic yield (58.91 mg PGE per g dry wt) compared to SCW without the addition of IL (39.27 mg PGE per g dry wt) at 175 °C. However, IL concentrations >0.25 M reduced yields due to a significant increase in solvent viscosity. Saravana et al.39 studied the addition of seven deep eutectic solvents (DESs) in SCW; component one of the DES was choline chloride, and component two was varied (1,2-propanediol, glycerol, ethylene glycol, 1,3-butanediol, 1,4-butanediol, urea, and propanedioic acid) at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio of component one to two. Choline chloride:glycerol showed the highest overall yield of alginate and fucoidan and was therefore used in subsequent optimization studies (temperature, pressure, solvent-to-solid ratio, and water content in DES). The optimized alginate (28.12 dry wt%) and fucoidan (14.93 dry wt%) yields were obtained at 150 °C, 19.85 bar, 70% water content in choline chloride: glycerol, and a solvent/solid ratio of 36.81 mL g−1. These results were higher than SCWE without DES, where yields were 70% lower for alginate (8.21 wt% dry basis) and 56% lower for fucoidan (6.52 wt% dry basis).
2 ratio of component one to two. Choline chloride:glycerol showed the highest overall yield of alginate and fucoidan and was therefore used in subsequent optimization studies (temperature, pressure, solvent-to-solid ratio, and water content in DES). The optimized alginate (28.12 dry wt%) and fucoidan (14.93 dry wt%) yields were obtained at 150 °C, 19.85 bar, 70% water content in choline chloride: glycerol, and a solvent/solid ratio of 36.81 mL g−1. These results were higher than SCWE without DES, where yields were 70% lower for alginate (8.21 wt% dry basis) and 56% lower for fucoidan (6.52 wt% dry basis).
7 Comparison of ASE and SCWE techniques
7.1 Similarities, differences, advantages, and disadvantages of ASE and SCWE
ASE and SCWE are two advanced pressurized techniques that can both use water at subcritical conditions; however, in ASE, the pressure is typically set at approximately 100 bar (system design), while in SCWE, the pressure is set by the system temperature (vapor pressure of water). In this context, ASE can be considered a form of SCWE, particularly when water is the sole solvent and conditions align with those of SCWE. Both ASE and SCWE are designed to enhance extraction efficiency, resulting in faster extraction rates, reduced solvent usage, and higher yields compared to conventional SLE methods. These two techniques aim to minimize harmful organic solvents, adhering to green chemistry principles. Although ASE and SCWE share some similarities, they are distinct methods with unique operational parameters and applications. Table 5 outlines the typical operating conditions of ASE compared to SCWE in terms of solvent usage, temperature and pressure conditions, average extraction time, and application scales.| ASE | SCWE | |
|---|---|---|
| Solvent | Solvents and/or water | Water, water and co-solvents |
| Temperature | 40–200 °C | 100–220 °C |
| Pressure | Approximately 100 bar | 5–80 bar |
| Average extraction time | 12–18 min | 10–60 min |
| Application scale | Lab extraction/characterization or small scale | From lab to industrial scale |
Despite their advantages, as outlined in Table 6, ASE and SCWE have some limitations. The analytical-scale ASE system has a high instrument cost and operates under very high-pressure conditions (approximately 100 bar), making it challenging to scale up.88 In contrast, SCW equipment operates at lower pressures (typically 5–80 bar) and is much simpler than ASE systems or supercritical fluids. Therefore, it is less expensive and more readily scalable for industrial applications.17,89 However, the main challenge of ASE and SCWE is the need for high temperatures, which can lead to the thermal degradation of heat-sensitive compounds. Additionally, SCW is more reactive and corrosive, potentially complicating processing control due to accelerated hydrolysis and oxidation of some compounds. Static SCW processes may increase the residence time of compounds compared to dynamic modes, making thermally labile compounds more susceptible to degradation.52 Moisture in the extraction solution is not always easily removed in SCWE, requiring additional processing such as separation, dehydration, and precipitation steps. Zhang et al.17 also noted that SCW equipment is not easy to clean, possibly due to potential char buildup.
| ASE | SCWE | |
|---|---|---|
| Advantages | - Fully automatic extraction and cleaning cycles | - Water as green, cheap, and readily available solvent |
| - Low solvent consumption (about 10–25 mL g−1) and extraction time (usually 12–18 min) | - Can use wet biomass, reducing drying costs and energy consumption | |
| - Suitable for a wide range of solvents | - Can extract polar, moderately polar, low-polar, and non-polar compounds separately | |
| - Possibility of extraction of 24 samples in one batch cycle | - Less expensive equipment than ASE systems | |
| - Higher efficiency than SLE | ||
| - Continuous operation possible | ||
| Disadvantages | - High equipment costs | - Moisture removal from extracts may require additional steps (e.g., separation, dehydration, precipitation) |
| - High-pressure settings make scaling up difficult | - Thermal degradation can occur at higher temperatures | |
| - Potential thermal degradation of heat-sensitive compounds | - Static SCWE may increase the residence time of compounds compared to dynamic modes, making thermally labile compounds more susceptible to degradation | |
| - Equipment is not easy to clean |
7.2 Economic and environmental sustainability perspective of ASE and SCWE
Pressurized liquid extraction using ASE and SCW extraction technologies offers several advantages over conventional solvent-based methods, particularly reducing the use of harmful organic solvents and producing less waste. Economic and environmental sustainability analysis of ASE and SCWE can give insights into beach-cast brown algae valorization design economically and environmentally friendly.ASE and SCWE can potentially lower operational costs by increasing extraction efficiency, reducing organic solvent consumption, management, disposal, and shortening extraction times. However, the initial investment in pressurized equipment can be significant. As developed by Dionex Corporation, ASE systems are designed for laboratory-scale extraction and offer automated extraction and cleaning cycles, saving on labor and time but high instrument costs.88 The high-pressure settings (approximately 100 bar) of the ASE system also make scale-up difficult and costly. In contrast, SCWE uses pressurized reactors and water as a solvent, which is cheaper and readily available. Vendors such as Parr Instrument Company, Amar Equipment, and Milestone Company provide pressurized vessels. SCWE can also process high-moisture or wet biomass without requiring energy-intensive drying operations, thereby reducing pre-treatment costs associated with drying.90 Furthermore, SCWE operates at water vapor pressure, is more readily scalable for industrial applications, and potentially offers long-term cost benefits than ASE systems.
Todd and Baroutian91 conducted a techno-economic comparison of SCW (125 °C and 100 bar), supercritical CO2 (40 °C and 150 bar), and conventional organic solvent extraction (50 °C) for phenolic compounds from grape marc. The techno-economic analysis showed that the cost of manufacture by SCWE (NZ$89.60/kg product) is comparable to the current solvent extraction techniques (NZ$87.00/kg product) but significantly lower than supercritical CO2 extraction (NZ$123.40/kg product). While the study assumes equal phenolic extraction capacity (25 mg GAE per mg dry wt feedstock) across all methods for simplicity, it acknowledges that actual yields may vary in practice. Thakhiew et al.92 also performed a techno-economic analysis comparing SCW hydrolysis and lipase-catalyzed hydrolysis for continuous fatty acid production, using palm oil splitting as a model. Their results indicated that SCW hydrolysis (350 °C and 200 bar) requires higher capital investment and energy consumption than lipase-catalyzed hydrolysis, while the annual operating costs for both methods are comparable.
Another techno-economic feasibility study93 on microalgal biofuel production suggested that incorporating biorefinery approaches, which valorize by-products and co-products, can improve overall economics. This contrasts with producing biofuel as a sole product, which is not economically viable. However, there is limited information on the economic assessment of SCWE application in brown algae valorization.94 Future case studies and experimental data at both lab and large scale are needed to establish the economic feasibility of beach-cast brown algae valorization using the SCW technique.
To evaluate and improve the environmental sustainability of ASE and SCWE techniques for beach-cast brown algae valorization, Life Cycle Analysis (LCA) provides valuable insights into the environmental impacts of different extraction methods, materials, and energy sources.95 Todd and Baroutian91 conducted a “gate-to-gate” analysis to quantify the potential environmental impact (PEI) of SCWE, supercritical CO2 extraction, and conventional organic solvent extraction methods using energy and material balances. They compared eight PEI categories: human toxicity by ingestion (HTPI), human toxicity by inhalation/dermal exposure (HTPE), terrestrial toxicity potential (TTP), aquatic toxicity potential (ATP), global warming potential (GWP), ozone depletion potential (ODP), photochemical oxidation potential (PCOP), and acidification potential (AP). The results indicated that supercritical CO2 had the highest PEIs (209 PEI per tonne product) and greenhouse gas emissions (11.8 kg CO2-equivalent per kg product), followed by SCWE with 178 PEI per tonne product and 10.0 kg CO2-equivalent per kg product and conventional solvent extraction with 175 PEI per tonne product and 9.8 kg CO2-equivalent per kg product. In another study,31 a comprehensive ex-ante “harbor-to-gate” LCA was conducted to evaluate the environmental sustainability of two biorefinery systems using the South African brown algae Ecklonia maxima. Two biorefinery systems co-produce alginate, laminarin, and fucoidan through SCWE (100 °C and 40 bar) and hot water extraction (HWE, 60 °C for 6 h) and were compared to an industrial-scale alginate production system (referred to as “REF”). Four impact categories were selected: climate change, mineral resource scarcity, marine eutrophication, and water consumption. The results showed that the brown algae biorefinery system using SCWE had a higher carbon footprint (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 665 kg CO2-equivalent per t dry matter feedstock) compared to HWE (13
665 kg CO2-equivalent per t dry matter feedstock) compared to HWE (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 530 kg CO2-equivalent per t dry matter feedstock) and the REF system (5188 kg CO2-equivalent per t dry matter feedstock). The main contributors were the electric energy consumption of the extraction process and spray drying in SCWE and HWE. The total electric energy consumption was 20 MW h per t dry matter feedstock for SCWE and 9.7 MW h per t for HWE. In SCWE and HWE, oven drying of alginate and spray drying of fucoidan and laminarin accounted for 61% and 77% of the total electric energy consumption, while the extraction process contributed 35% and 18%, respectively.
530 kg CO2-equivalent per t dry matter feedstock) and the REF system (5188 kg CO2-equivalent per t dry matter feedstock). The main contributors were the electric energy consumption of the extraction process and spray drying in SCWE and HWE. The total electric energy consumption was 20 MW h per t dry matter feedstock for SCWE and 9.7 MW h per t for HWE. In SCWE and HWE, oven drying of alginate and spray drying of fucoidan and laminarin accounted for 61% and 77% of the total electric energy consumption, while the extraction process contributed 35% and 18%, respectively.
These findings demonstrated that SCWE was not the most holistically eco-friendly method due to its large energy consumption. Four scenarios were further analyzed to explore process optimization opportunities and improve environmental performance: side-stream valorization, optimized product drying process, adoption of a greener electricity mix, and use of more resilient stainless steel.31 Among these scenarios, the side-stream valorization applied zero waste and closed-loop resource management principles by integrating solid and liquid-side stream valorization, showing overall improvements. The solid-side stream was used for feed supplement production, and the liquid-side stream was passed through a reverse osmosis (PO) membrane to produce liquid fertilizer and recover water. Additionally, using electricity from more renewable sources rather than coal can further enhance the sustainability of the SCWE system.
8 Extraction kinetics and modeling of bioactive compounds using ASE and SCWE from beach-cast brown algae
ASE and SCWE methods offer an alternative to conventional SLE, making the study of extraction mechanisms and modeling essential for scale-up. This section provides insights into the current status of extraction rate/kinetic models and scale-up for extracting bioactive compounds from beach-cast brown algae using ASE and SCWE.8.1 Design and scale-up status of ASE and SCWE
As noted, ASE operates at fixed high pressures (around 100 bar) to facilitate automatic solvent filling and purging, allowing the solvent (organic or aqueous) to penetrate solid matrices and operate in a fixed bed design. In contrast, SCWE maintains pressure at/slightly above water's vapor pressure, ensuring water remains liquid within designated temperature ranges. The subcritical region for water is characterized by temperatures of 100–374 °C and pressures of 1–221 bar,17,96 as depicted in Fig. 4. Most SCWE studies on brown algae use batch reactors focusing on lower temperatures and pressures, between 100–220 °C and 5–80 bar, for bioactive compound extraction (Table 4). The pressurized extraction system comprises key components:52,97 solvent supply, solvent transport pump, heater, pressure vessel, condenser, and collection vessel. Depending on the cost and specific applications, detailed design may have additional features such as autosamplers, gas purge systems, pressure regulators, safety features, and integration with downstream separation systems.Although SCWE is more readily to scale up compared to the ASE system, most research on SCWE for bioactive compound extraction remains at the laboratory scale, with few pilot-scale studies reported.16 Kwon and Chung98 studied the scale-up of a lab-scale 22 mL ASE system (packed bed, 23 mm i.d. × 50 mm long, 1 g sample size with particle size < 10 mm) to a larger 8 L SCW system (stirring pressurized reactor, 143 mm i.d. × 520 mm long, 250 g sample size). They studied turmeric as the solid feedstock to extract curcuminoids. Results showed comparable yields between lab-scale (15.80 dry wt%) and 8 L (13.58 dry wt%) under optimized conditions (135 °C, 5 min isothermal extraction time, and 50% ethanol in water). Varying pressure using nitrogen (from 5 to 100 bar) in the 8 L system did not significantly affect curcuminoid yield (13.50–13.58 dry wt%). This is likely due to minimal changes in water's dielectric constant and density within the pressure range at studied temperatures. In another study, Ko et al.99 assessed the scaling up of ASE with water as a solvent for flavonoid extraction from agricultural by-product satsuma mandarin peel using the same size, 22 mL lab-scale packed-type ASE and 8 L stirring-type system. The yields of flavonoids obtained at the lab scale (117.80 mg per g dry wt) and 8 L system (113.40 mg per g dry wt) were similar under optimized conditions (130 °C, 15 min isothermal extraction time, and water-to-solid ratio of 34 mL g−1). The above two studies indicate that rate-limiting phenomena common in scale-up (such as poor contact due to fluid dynamics, external mass transfer, etc.) were not encountered; however, the scale of the larger system in 8 L is still relatively small.
To assess the scale-up of batch SCW systems from laboratory (500 mL) to 25 L scale, Trigueros et al.96 studied the valorization of red algae residue post-agar extraction. SCW treatment was conducted at 175 °C, 20 bar, and 5% (w/v) biomass loading in both lab and 25 L scale systems. The larger system yields were consistently lower for monomer, oligomer, and free amino acids but approximately the same for protein under the same conditions. At lab scale, oligomer yield was 78.6%, monomer at 4.6%, and protein of 37.5% with 17.8 mg free amino acids/g protein compared to 25 L scale with oligomer at 71.4%, monomer at 3.0%, and protein at 37.4% with 14.2 mg free amino acids per g protein. The phenolic yield was 45% higher on the lab scale (17.9 g GAE per kg dry wt) compared to 25 L (10.0 g GAE per kg dry wt) at 175 °C. In another study, Alonso-Riaño et al.85 compared the valorization of brewer's spent grain at the same sizes, 500 mL and 25 L system. As in the previous study, maximum yields were similar in both systems. At the 25 L scale (170 °C and 22 min isothermal time), total carbohydrates were 56%, total pentose was 78%, and protein was 64% with 21 mg free amino acids/g protein. The 500 mL lab-scale system yielded a maximum phenolic content of 34 mg GAE/g dry wt, 38% higher than the pilot system. However, the lab-scale operating conditions were not truly comparable to the 25 L system. The lab system was at 174 °C, 15.7 min ramp-up time with slow heating rate from 3–14.4 °C min−1, 45 min isothermal time, 60 min cooling time, and 50 bar, while the pilot conditions were the similar temperature but faster heating rate (13.4–83.4 °C min−1) with 4.5 min ramp-up time to reach desired temperature of 170 °C, half of the isothermal time (22 min), and lower pressure (20 bar).
Moral et al.5 explored the valorization potential of tidal waste biomass from the Andalusian Mediterranean coastline using a 15 L-scale SCW batch reactor. The waste feedstock comprised beach-cast brown algae (Dyctiota dichotoma) and seagrass (Posidonia oceanica and Zostera noltii). An optimal mixture yield of glucose (2.29 g L−1), xylose (3.11 g L−1), and arabinose (1.27 g L−1) were obtained at a temperature of 150 °C, 30 min isothermal reaction time (without consideration of ramp-up time), and a liquid-to-solid ratio of 8 mL g−1.
8.2 Challenges for large-scale application
Although the above studies demonstrate the potential of SCWE for larger-scale applications, there are limited reports on scaling up bioactive compound extraction from beach-cast brown algae using SCWE. Developing SCW equipment for large-scale use is still in its early stages and presents significant challenges. Key issues include ensuring safety, managing energy consumption, and maintaining the yield and bioactivity of the extracts. Legal regulations are also necessary to establish production standards and standardized usage rules for SCW equipment. Addressing these issues is crucial to overcoming the safety and energy consumption challenges associated with large-scale SCW equipment.100In order to maintain the yield and quality of extract, one of the main limitations in scaling up is achieving uniform mass and heat transfer. In laboratory settings, small-scale reactors using small sample sizes allow for better control of heat (ramp-up) and mass (mixing) transfer resistances, mitigating issues related to ramp-up time and ensuring consistent reaction conditions. However, as the process scales up, maintaining uniform heat and reactant/product distribution becomes challenging. Ensuring rapid ramp-up rates so that the bulk material reaches the desired temperature quickly is difficult, as larger volumes introduce heat transfer resistances. This discrepancy can lead to variations in the reaction time, where some compounds may initiate reactions at lower temperatures, leading to potential decomposition or hydrolysis before reaching the target conditions.
Laboratory work with small-scale reactors is essential for identifying the key parameters needed for scaling up processes, often involving the development of kinetic models.101 However, scaling up introduces complexities in maintaining these parameters, especially in terms of heat and mass transfer. Therefore, further research and development are needed to optimize reactor designs and process controls for large-scale applications.
8.3 Mathematical modeling
In modeling the extraction rate of bioactive compounds in ASE/SCWE, the mechanism is collapsed from the five stages outlined above to two stages:52 (i) a washing stage and (ii) a diffusion stage. The assumption is that the system used to study kinetics is using a small enough sample size that external mass transfer resistances can be ignored or collapsed into the diffusion stage. The washing stage is described as solvent penetration into the porous solids, exposing the solid surface to the solvent, resulting in the rapid dissolution of compounds. In the diffusion stage, compounds diffuse from the interior of the solids to the external surface, followed by the mass transfer of solutes from the solid surface into the bulk solvent.From an engineering perspective, rate models are crucial tools for process optimization, system control, and scaling up.101 Although numerous kinetic models are widely used to extract compounds from plant biomass, studies on marine algae biomass remain relatively limited. Commonly used models for investigating the extraction kinetics of bioactive compounds in ASE/SCWE include Fick's law and empirical models such as first-order kinetic, second-order kinetic, two-site kinetic, and Peleg's models (Table 7).
| Raw material | Bioactive compounds | Extraction technique | Type of model | Equation | Ref. |
|---|---|---|---|---|---|
| Spent coffee grounds | Oil | ASE | Two-site kinetic | Eqn (10) | 102 |
| Fick's law | Eqn (4) | ||||
| Arrhenius equation | Eqn (6) | ||||
| Cocoa bean shell | Fat | ASE | Fick's law | Eqn (4) | 103 |
| Arrhenius equation | Eqn (6) | ||||
| Watermelon seed | Oil | ASE | Second-order kinetic | Eqn (9) | 104 |
| Peleg's | Eqn (12) | ||||
| Fick's law | Eqn (4) | ||||
| Cocoa bean shell | Flavanols | ASE | Peleg's | Eqn (12) | 105 |
| Pomelo peels | Pectin | SCWE | First-order kinetic | Eqn (8) | 106 |
| Noni fruits | Scopoletin, alizarin, rutin | SCWE | Two-site kinetic | Eqn (10) | 107 |
| Grape skins and defatted grape seeds | Polyphenols | SCWE | Two-site kinetic | Eqn (10) | 108 |
| Loquat leaves | Lipids | SCWE | Second-order kinetic | Eqn (9) | 109 |
| Grape canes | Trans-resveratrol | SCWE | Fick's law | Eqn (3) | 110 |
| Arrhenius equation | Eqn (6) |
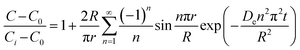 | (1) |
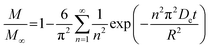 | (2) |
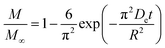 | (3) |
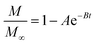 | (4) |
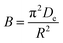 | (5) |
Fick's law-based solutions are commonly used to estimate De of solutes in porous solid samples. These models have found application in various extraction processes, including ASE of oil from spent coffee grounds,102 fat extraction from cocoa bean shells in the ASE process,103 and SCWE of trans-resveratrol from grape canes.110 As discussed in previous sections, temperature is the most important parameter in ASE/SCWE. To investigate temperature impact on the extraction process, the Arrhenius equation eqn (6) has been used to describe the De as a function of temperature.112
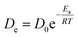 | (6) |
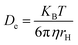 | (7) |
8.3.2.1 First-order kinetic model. The first-order kinetic model, eqn (8), has been applied to describe bioactive compound extraction with assumptions of uniform solute distribution. The model provides the fraction of bioactive compound extracted (Ct/C∞) at time t. However, the first-order kinetic model fails to account for equilibrium and is valid only when solute concentrations are below the saturation.106
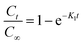 | (8) |
8.3.2.2 Second-order kinetic model. The second-order kinetic model eqn (9) assumes the solute concentration will reach equilibrium in the solvent phase for a given set of conditions.
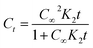 | (9) |
8.3.2.3 Two-site kinetic model. The two-site kinetic model developed by So and MacDonald115 assumes two distinct phases involved in the extraction process where the fast washing step and a slow diffusion step occur simultaneously. The combination of two steps is expressed by the two-site first-order equation, eqn (10).
| Ct = Cw[1 − exp(−Kwt)] + Cd[1 − exp(−Kdt)] | (10) |
8.3.2.4 Peleg's model. Peleg's model is a well-known kinetic model based on the second-order kinetic model, as shown in eqn (11).116
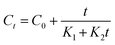 | (11) |
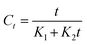 | (12) |
The two-site kinetic model has been successfully applied in ASE and SCWE of bioactive compounds from various biomass sources, including spent coffee grounds,102 noni fruits,107 and grape skins/defatted grapes.108 Toda et al.102 observed that the washing rate (Kw) was 27 times higher than the diffusion rate (Kd) in ASE with absolute ethanol solvent in batch mode at an extraction temperature of 150 °C. In another dynamic SCWE study on the recovery of low methoxyl pectin from pomelo peels,106 the first-order kinetic model exhibited better agreement with experimental data (R2 > 0.94) than the two-site kinetic model, which failed to fit the data and reverted to the first-order kinetic model. This suggests that the distinction between “fast” and “slow” kinetics is not significant in the pectin SCW extraction process in dynamic mode, and the first-order kinetic model is more appropriate in this case. The second-order kinetic model was effectively used to describe watermelon seed oil extraction in ASE, demonstrating a better fitting (R2 > 0.99) compared to Peleg's model (R2 > 0.98) and Fick's model (R2 > 0.85) at temperatures of 60–80 °C.104 The second-order kinetic model was also used to model lipids extraction from loquat leaves in dynamic SCWE, showing good agreement between the estimated data and the experimental data (R2 > 0.98).109 Peleg's model found application in representing the ASE batch mode for extracting oil from watermelon seeds104 and flavanols from cocoa bean shells,105 providing a satisfactory fit for the experimental data. Both the second-order kinetic model in eqn (9) and Peleg's model in eqn (12) are hyperbolic equations suitable for estimating the initial extraction rate and maximum extraction yield.
9 Conclusion and future trends
This work provides a comprehensive review of pressurized liquid processes (ASE and SCWE) as a “green” alternative to conventional SLE for extracting bioactive compounds (alginate, fucoidan, laminarin, phenolics, and fucoxanthin) from beach-cast brown algae. These bioactive compounds have broad applications in the food, cosmetic, and pharmaceutical industries. ASE and SCWE are potentially more sustainable extraction processes compared to conventional SLE in the elimination of harmful organic solvent use, ease in reusing and/or treating the remaining solids, and ease of reuse of produced water. This review compares and outlines the advantages and disadvantages of these two advanced pressurized techniques. ASE is more suited for lab-scale characterization and analysis, while SCWE has better potential for scale-up to production applications. In general, these pressurized techniques can extract key valuable products more efficiently and selectively by modifying process conditions (mainly solvent combinations and temperatures) while minimizing extraction time and chemical usage compared to conventional SLE. However, the higher temperature required in SCWE means costs (environmental and financial) in energy consumption must be balanced against the costs associated with SLE (e.g., waste generation, handling, and consumption of toxic solvents) in larger-scale applications. Work in process optimization, side-stream valorization, and adopting a greener electricity mix would improve the balance in favor of SCWE.SCWE is more readily scalable than ASE (which is mainly for analytical analysis). However, the bulk of the SCWE work is at the lab scale, with limited scale-up work. While lab scale work is critical for establishing extraction rates, it does not effectively capture the impact transport phenomena (heat and mass transfer effects) will have on overall reaction time (i.e., time material spends at reaction temperature or temperature variability within reactor) and subsequent reactor size and operating conditions. Future research studies should focus on the feasibility of large-scale operation and industrial equipment design, optimizing parameters such as solvent combinations, temperature, and total extraction time (considering ramp-up, isothermal, and cooling time) to balance quantity and quality. This includes establishing kinetic models and incorporating mass and heat transfer models. Such research initiatives are hoped to significantly contribute to the understanding, advancement, and future applications of high-value products obtained from beach-cast brown algae using ASE and SCWE, supporting circular economic developments.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
Yu Zhang: conceptualization, visualization, writing – original draft & review & editing. Kelly Hawboldt: supervision, funding acquisition, project administration, conceptualization, writing – review & editing. Stephanie MacQuarrie: writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Government of Canada's New Frontiers in Research Fund (NFRF), housed within the Social Sciences and Humanities Research Council (SSHRC).References
- V. Rudovica, A. Rotter, S. P. Gaudêncio, L. Novoveská, F. Akgül, L. K. Akslen-Hoel, D. A. Alexandrino, O. Anne, L. Arbidans and M. Atanassova, Front. Mar. Sci., 2021, 8, 723333 CrossRef.
- T. B. Harb and F. Chow, Algal Res., 2022, 62, 102643 CrossRef.
- S. Pardilhó, J. Cotas, L. Pereira, M. B. Oliveira and J. M. Dias, Biotechnol. Adv., 2022, 60, 107987 CrossRef PubMed.
- A. Mandalka, M. I. L. G. Cavalcanti, T. B. Harb, M. Toyota Fujii, P. Eisner, U. Schweiggert-Weisz and F. Chow, Foods, 2022, 11, 1201 CrossRef CAS PubMed.
- A. Moral, V. Greyer, R. Aguado and A. Tijero, Biomass Bioenergy, 2023, 174, 106856 CrossRef CAS.
- FAO, The State of World Fisheries and Aquaculture, FAO, Rome, 2024 Search PubMed.
- E. T. Kostas, J. M. Adams, H. A. Ruiz, G. Durán-Jiménez and G. J. Lye, Renewable Sustainable Energy Rev., 2021, 151, 111553 CrossRef CAS.
- A. Gan and S. Baroutian, J. Chem. Technol. Biotechnol., 2022, 97, 1929–1940 CrossRef CAS.
- S. Ghosh, T. Sarkar, S. Pati, Z. A. Kari, H. A. Edinur and R. Chakraborty, Front. Mar. Sci., 2022, 9, 832957 CrossRef.
- A. Silva, L. Cassani, C. Grosso, P. Garcia-Oliveira, S. L. Morais, J. Echave, M. Carpena, J. Xiao, M. F. Barroso and J. Simal-Gandara, Crit. Rev. Food Sci. Nutr., 2024, 64, 1283–1311 CrossRef CAS PubMed.
- E. Quitério, C. Grosso, R. Ferraz, C. Delerue-Matos and C. Soares, Mar. Drugs, 2022, 20, 677 CrossRef PubMed.
- Y. P. Didion, T. G. Tjalsma, Z. Su, M. Malankowska and M. Pinelo, Sep. Purif. Technol., 2023, 124147 CrossRef.
- A. Perez-Vazquez, M. Carpena, P. Barciela, L. Cassani, J. Simal-Gandara and M. A. Prieto, Antioxidants, 2023, 12, 612 CrossRef CAS PubMed.
- M. M. Poojary, A. Laurora, M. N. Lund and B. K. Tiwari, in Innovative and Emerging Technologies in the Bio-Marine Food Sector, Elsevier, 2022, pp. 441–479 Search PubMed.
- R. Carabias-Martínez, E. Rodríguez-Gonzalo, P. Revilla-Ruiz and J. Hernández-Méndez, J. Chromatogr. A, 2005, 1089, 1–17 CrossRef PubMed.
- T. Majeed, I. Shabir, S. Srivastava, N. Maqbool, A. H. Dar, K. Jan, V. K. Pandey, R. Shams, I. Bashir and K. K. Dash, Trends Food Sci. Technol., 2023, 104316 Search PubMed.
- J. Zhang, C. Wen, H. Zhang, Y. Duan and H. Ma, Trends Food Sci. Technol., 2020, 95, 183–195 CrossRef CAS.
- C. Jacobsen, A.-D. M. Sørensen, S. L. Holdt, C. C. Akoh and D. B. Hermund, Annu. Rev. Food Sci. Technol., 2019, 10, 541–568 CrossRef CAS PubMed.
- S. U. Kadam, B. K. Tiwari and C. P. O'Donnell, J. Agric. Food Chem., 2013, 61, 4667–4675 CrossRef CAS PubMed.
- I. Michalak and K. Chojnacka, Eng. Life Sci., 2015, 15, 160–176 CrossRef CAS.
- E. A. Flores-Contreras, R. G. Araújo, A. A. Rodríguez-Aguayo, M. Guzmán-Román, J. C. García-Venegas, E. F. Nájera-Martínez, J. E. Sosa-Hernández, H. M. Iqbal, E. M. Melchor-Martínez and R. Parra-Saldivar, Plants, 2023, 12, 2445 CrossRef CAS PubMed.
- D. Pal and W. Hogland, J. Environ. Manage., 2022, 302, 113971 CrossRef CAS PubMed.
- M. A. Masri, S. Younes, M. Haack, F. Qoura, N. Mehlmer and T. Brück, Energy Technol., 2018, 6, 1026–1038 CrossRef CAS.
- J. J. Milledge and P. J. Harvey, J. Mar. Sci. Eng., 2016, 4, 60 CrossRef.
- S. Angus, Scott. Geogr. J., 2017, 133, 101–114 CrossRef.
- M. D. Guiry and L. Morrison, J. Appl. Phycol., 2013, 25, 1823–1830 CrossRef.
- C. Bertagnolli, A. P. D. Espindola, S. J. Kleinübing, L. Tasic and M. G. C. da Silva, Carbohydr. Polym., 2014, 111, 619–623 CrossRef CAS PubMed.
- A. Lorbeer, R. Tham and W. Zhang, J. Appl. Phycol., 2013, 25, 717–732 CrossRef.
- S. U. Kadam, B. K. Tiwari and C. P. O'Donnell, Eur. J. Phycol., 2015, 50, 24–31 CAS.
- M. García-Vaquero, G. Rajauria, J. V. O'Doherty and T. Sweeney, Food Res. Int., 2017, 99, 1011–1020 CrossRef PubMed.
- X. Zhang, A. Border, N. Goosen and M. Thomsen, Algal Res., 2021, 58, 102348 CrossRef.
- M. Mac Monagail, L. Cornish, L. Morrison, R. Araújo and A. T. Critchley, Eur. J. Phycol., 2017, 52, 371–390 CrossRef.
- B. L. Tagliapietra and M. T. P. S. Clerici, Food Res. Int., 2023, 167, 112655 CrossRef CAS PubMed.
- B. K. Tiwari and D. J. Troy, in Seaweed Sustainability, Elsevier, 2015, pp. 1–6 Search PubMed.
- L. Pereira, L. Morrison, P. S. Shukla and A. T. Critchley, J. Appl. Phycol., 2020, 32, 3561–3584 CrossRef CAS.
- S. Saji, A. Hebden, P. Goswami and C. Du, Sustainability, 2022, 14, 5181 CrossRef CAS.
- A. Dobrinčić, S. Balbino, Z. Zorić, S. Pedisić, D. Bursać Kovačević, I. Elez Garofulić and V. Dragović-Uzelac, Mar. Drugs, 2020, 18, 168 CrossRef PubMed.
- T. M. Aida, Y. Kumagai and R. L. Smith Jr, J. Bioresour. Bioprod., 2022, 7, 173–179 CrossRef CAS.
- P. S. Saravana, Y.-N. Cho, H.-C. Woo and B.-S. Chun, J. Cleaner Prod., 2018, 198, 1474–1484 CrossRef CAS.
- P. S. Saravana, Y.-J. Cho, Y.-B. Park, H.-C. Woo and B.-S. Chun, Carbohydr. Polym., 2016, 153, 518–525 CrossRef CAS PubMed.
- T. Hahn, S. Lang, R. Ulber and K. Muffler, Process Biochem., 2012, 47, 1691–1698 CrossRef CAS.
- S. A. Foley, E. Szegezdi, B. Mulloy, A. Samali and M. G. Tuohy, J. Nat. Prod., 2011, 74, 1851–1861 CrossRef CAS PubMed.
- G. Kopplin, A. M. Rokstad, H. Mélida, V. Bulone, G. Skjåk-Bræk and F. L. Aachmann, ACS Appl. Bio Mater., 2018, 1, 1880–1892 CrossRef CAS PubMed.
- E. Sanniyasi, R. K. Gopal, R. Damodharan, A. Arumugam, M. Sampath Kumar, N. Senthilkumar and M. Anbalagan, Sci. Rep., 2023, 13, 14452 CrossRef CAS PubMed.
- R. E. Abraham, P. Su, M. Puri, C. L. Raston and W. Zhang, Algal Res., 2019, 38, 101389 CrossRef.
- E. T. Kostas, D. A. White and D. J. Cook, Algal Res., 2017, 28, 211–219 CrossRef.
- Y. Yuan and D. J. Macquarrie, Bioresour. Technol., 2015, 198, 819–827 CrossRef CAS PubMed.
- A. J. Lorbeer, S. Charoensiddhi, J. Lahnstein, C. Lars, C. M. Franco, V. Bulone and W. Zhang, J. Appl. Phycol., 2017, 29, 1515–1526 CrossRef CAS.
- V. Subbiah, F. Ebrahimi, O. T. Agar, F. R. Dunshea, C. J. Barrow and H. A. Suleria, Sci. Rep., 2024, 14, 4335 CrossRef CAS PubMed.
- Y.-X. Li, I. Wijesekara, Y. Li and S.-K. Kim, Process Biochem., 2011, 46, 2219–2224 CrossRef CAS.
- N. Kumar and N. Goel, Biotechnol. Rep., 2019, 24, e00370 CrossRef PubMed.
- H. Dominguez and M. J. G. Muñoz, Water Extraction of Bioactive Compounds: from Plants to Drug Development, Elsevier, 2017 Search PubMed.
- C. Lourenço-Lopes, M. Fraga-Corral, C. Jimenez-Lopez, M. Carpena, A. Pereira, P. García-Oliveira, M. Prieto and J. Simal-Gandara, Trends Food Sci. Technol., 2021, 117, 163–181 CrossRef.
- I. Fatima, M. Munir, R. Qureshi, U. Hanif, N. Gulzar and A. A. Sheikh, Crit. Rev. Food Sci. Nutr., 2023, 1–18 CrossRef PubMed.
- N. A. S. Din, A. S. Mohd Alayudin, N.-S. Sofian-Seng, H. A. Rahman, N. S. Mohd Razali, S. J. Lim and W. A. Wan Mustapha, Foods, 2022, 11, 2235 CrossRef CAS PubMed.
- C. Turner and M. Waldebäck, in Separation, Extraction and Concentration Processes in the Food, Beverage and Nutraceutical Industries, Elsevier, 2013, pp. 39–70 Search PubMed.
- T. B. Harb, M. S. Pereira, M. I. L. Cavalcanti, M. T. Fujii and F. Chow, J. Appl. Phycol., 2021, 33, 3383–3395 CrossRef CAS.
- A. Silva, C. Rodrigues, P. Garcia-Oliveira, C. Lourenço-Lopes, S. A. Silva, P. Garcia-Perez, A. P. Carvalho, V. F. Domingues, M. F. Barroso and C. Delerue-Matos, Foods, 2021, 10, 1915 CrossRef CAS PubMed.
- N. Heffernan, T. J. Smyth, R. J. FitzGerald, A. Soler-Vila and N. Brunton, Eur. J. Phycol., 2014, 49, 1765–1772 CAS.
- S. Periaswamy Sivagnanam, S. Yin, J. H. Choi, Y. B. Park, H. C. Woo and B. S. Chun, Mar. Drugs, 2015, 13, 3422–3442 CrossRef PubMed.
- L. Chen, Y. Wang, H. Yang, H. Li, W. Xu, G. Chen and H. Zhu, Molecules, 2018, 23, 1912 CrossRef PubMed.
- Y. Sun, S. Hou, S. Song, B. Zhang, C. Ai, X. Chen and N. Liu, Int. J. Biol. Macromol., 2018, 112, 985–995 CrossRef CAS PubMed.
- T. B. Harb, J. Vega, J. Bonomi-Barufi, V. Casas, R. Abdala-Diaz, F. L. Figueroa and F. Chow, Waste Biomass Valorization, 2023, 14, 2249–2265 CrossRef CAS.
- N. Peasura, N. Laohakunjit, O. Kerdchoechuen and S. Wanlapa, Int. J. Biol. Macromol., 2015, 81, 912–919 CrossRef CAS PubMed.
- B. E. Richter, B. A. Jones, J. L. Ezzell, N. L. Porter, N. Avdalovic and C. Pohl, Anal. Chem., 1996, 68, 1033–1039 CrossRef CAS.
- B. Khongthaw, P. Chauhan, K. Dulta, V. Kumar and J. O. Ighalo, J. Food Meas. Charact., 2023, 17, 1317–1342 CrossRef.
- A. T. Getachew, S. L. Holdt, A. S. Meyer and C. Jacobsen, Mar. Drugs, 2022, 20, 263 CrossRef CAS PubMed.
- A. Dobrinčić, S. Pedisić, Z. Zorić, M. Jurin, M. Roje, R. Čož-Rakovac and V. Dragović-Uzelac, Foods, 2021, 10, 1481 CrossRef PubMed.
- M. Plaza, S. Santoyo, L. Jaime, G. G.-B. Reina, M. Herrero, F. J. Señoráns and E. Ibáñez, J. Pharm. Biomed. Anal., 2010, 51, 450–455 CrossRef CAS PubMed.
- S. Santoyo, M. Plaza, L. Jaime, E. Ibañez, G. Reglero and J. Señorans, J. Appl. Phycol., 2011, 23, 909–917 CrossRef CAS.
- G. A. Sumampouw, C. Jacobsen and A. T. Getachew, J. Appl. Phycol., 2021, 33, 1195–1207 CrossRef CAS.
- A. d. P. Sánchez-Camargo, L. Montero, V. Stiger-Pouvreau, A. Tanniou, A. Cifuentes, M. Herrero and E. Ibáñez, Food Chem., 2016, 192, 67–74 CrossRef PubMed.
- Y. F. Shang, S. M. Kim, W. J. Lee and B.-H. Um, J. Biosci. Bioeng., 2011, 111, 237–241 CrossRef CAS PubMed.
- P. Otero, M. I. López-Martínez and M. R. García-Risco, J. Pharm. Biomed. Anal., 2019, 164, 86–92 CrossRef CAS PubMed.
- M. Castro-Puyana, M. Herrero, J. Mendiola and E. Ibáñez, in Functional Ingredients from Algae for Foods and Nutraceuticals, Elsevier, 2013, pp. 534–560 Search PubMed.
- Y. Cheng, F. Xue, S. Yu, S. Du and Y. Yang, Molecules, 2021, 26, 4004 CrossRef CAS PubMed.
- H. Cernadas, N. Flórez-Fernández, M. J. González-Muñoz, H. Domínguez and M. D. Torres, Food Bioprod. Process., 2019, 117, 275–286 CrossRef CAS.
- N. Flórez-Fernández, M. D. Torres, M. J. González-Muñoz and H. Domínguez, Food Chem., 2019, 277, 353–361 CrossRef PubMed.
- A. Gan and S. Baroutian, J. Supercrit. Fluids, 2022, 190, 105732 CrossRef CAS.
- M. Alboofetileh, M. Rezaei, M. Tabarsa, S. You, F. Mariatti and G. Cravotto, Int. J. Biol. Macromol., 2019, 128, 244–253 CrossRef CAS PubMed.
- M. Alboofetileh, M. Rezaei, M. Tabarsa, M. Rittà, M. Donalisio, F. Mariatti, S. You, D. Lembo and G. Cravotto, Int. J. Biol. Macromol., 2019, 124, 131–137 CrossRef CAS PubMed.
- A. Bordoloi and N. J. Goosen, J. Appl. Phycol., 2020, 32, 2307–2319 CrossRef CAS.
- P. S. Saravana, A. Tilahun, C. Gerenew, V. D. Tri, N. H. Kim, G.-D. Kim, H.-C. Woo and B.-S. Chun, J. Appl. Phycol., 2018, 30, 579–590 CrossRef CAS.
- T. V. Dinh, P. S. Saravana, H. C. Woo and B. S. Chun, Sep. Purif. Technol., 2018, 196, 287–299 CrossRef CAS.
- P. Alonso-Riaño, C. Ramos, E. Trigueros, S. Beltrán and M. Sanz, Ind. Crops Prod., 2023, 191, 115927 CrossRef.
- C. Pronyk and G. Mazza, J. Food Eng., 2009, 95, 215–226 CrossRef CAS.
- H. S. Na, J. Y. Kim, J. S. Park, G. C. Choi, S. I. Yang, J. H. Lee, J. Y. Cho and S. J. Ma, Korean J. Food Preserv., 2014, 21, 62–68 CrossRef.
- H. Giergielewicz-Możajska, Ł. Dąbrowski and J. Namieśnik, Crit. Rev. Anal. Chem., 2001, 31, 149–165 CrossRef.
- C. C. Teo, S. N. Tan, J. W. H. Yong, C. S. Hew and E. S. Ong, J. Chromatogr. A, 2010, 1217, 2484–2494 CrossRef CAS PubMed.
- S. Thiruvenkadam, S. Izhar, H. Yoshida, M. K. Danquah and R. Harun, Appl. Energy, 2015, 154, 815–828 CrossRef CAS.
- R. Todd and S. Baroutian, J. Cleaner Prod., 2017, 158, 349–358 CrossRef CAS.
- P. Thakhiew, C. Kesornpun, T. Wongnate, W. Kiatkittipong and N. Weeranoppanant, ACS Sustain. Chem. Eng., 2022, 10, 14724–14734 CrossRef CAS.
- G. V. Subhash, M. Rajvanshi, G. R. K. Kumar, U. S. Sagaram, V. Prasad, S. Govindachary and S. Dasgupta, Bioresour. Technol., 2022, 343, 126155 CrossRef PubMed.
- K. González-Gloria, R. M. Rodríguez-Jasso, E. Aparicio, M. L. C. González, E. T. Kostas and H. A. Ruiz, Bioresour. Technol. Rep., 2021, 16, 100863 CrossRef.
- M. Z. Hauschild, R. K. Rosenbaum and S. I. Olsen, Life Cycle Assessment, Springer, 2018 Search PubMed.
- E. Trigueros, C. Ramos, P. Alonso-Riaño, S. Beltrán and M. T. Sanz, Ind. Eng. Chem. Res., 2023, 62, 3503–3514 CrossRef CAS PubMed.
- A. H. Asl and M. Khajenoori, Mass Transfer-Advances in Sustainable Energy and Environment Oriented Numerical Modeling, 2013, 459–487 Search PubMed.
- H.-L. Kwon and M.-S. Chung, Food Chem., 2015, 185, 58–64 CrossRef CAS PubMed.
- M.-J. Ko, H.-L. Kwon and M.-S. Chung, Innovative Food Sci. Emerging Technol., 2016, 38, 175–181 CrossRef CAS.
- S. Ügdüler, K. M. Van Geem, M. Roosen, E. I. Delbeke and S. De Meester, Waste Manage., 2020, 104, 148–182 CrossRef PubMed.
- C.-H. Chan, R. Yusoff and G.-C. Ngoh, Chem. Eng. Res. Des., 2014, 92, 1169–1186 CrossRef CAS.
- T. A. Toda, P. d. C. Franco Visioli, A. L. de Oliveira and C. E. da Costa Rodrigues, J. Supercrit. Fluids, 2021, 177, 105332 CrossRef CAS.
- D. C. G. Okiyama, I. D. Soares, T. A. Toda, A. L. Oliveira and C. E. C. Rodrigues, Ind. Crops Prod., 2019, 130, 96–103 CrossRef CAS.
- J. Colivet, A. L. Oliveira and R. A. Carvalho, Sep. Purif. Technol., 2016, 169, 187–195 CrossRef CAS.
- D. C. Okiyama, I. D. Soares, M. S. Cuevas, E. J. Crevelin, L. A. Moraes, M. P. Melo, A. L. Oliveira and C. E. Rodrigues, Food Res. Int., 2018, 114, 20–29 CrossRef CAS PubMed.
- S. Q. Liew, W. H. Teoh, C. K. Tan, R. Yusoff and G. C. Ngoh, Int. J. Biol. Macromol., 2018, 116, 128–135 CrossRef CAS PubMed.
- R. Jamaludin, D.-S. Kim, L. M. Salleh and S.-B. Lim, Foods, 2021, 10, 2260 CrossRef CAS PubMed.
- K. S. Duba, A. A. Casazza, H. B. Mohamed, P. Perego and L. Fiori, Food Bioprod. Process., 2015, 94, 29–38 CrossRef CAS.
- E. Mlyuka, S. Zhang, L. Wang, Z. Zheng and J. Chen, Int. J. Food Eng., 2016, 12, 547–555 CrossRef CAS.
- E. Karacabey, G. Mazza, L. Bayındırlı and N. Artık, Food Bioprocess Technol., 2012, 5, 359–371 CrossRef CAS.
- J. Crank, The Mathematics of Diffusion, Oxford University Press, 1979 Search PubMed.
- E. E. Perez, A. A. Carelli and G. H. Crapiste, J. Food Eng., 2011, 105, 180–185 CrossRef CAS.
- J. Benitez, Principles and Modern Applications of Mass Transfer Operations, John Wiley & Sons, 2011 Search PubMed.
- I. Das and A. Arora, Food Hydrocolloids, 2021, 120, 106931 CrossRef CAS.
- G. C. So and D. G. MacDonald, Can. J. Chem. Eng., 1986, 64, 80–86 CrossRef.
- M. Peleg, J. Food Sci., 1988, 53, 1216–1217 CrossRef.
| This journal is © The Royal Society of Chemistry 2024 |
