N/P-doped NiFeV oxide nanosheets with oxygen vacancies as an efficient electrocatalyst for the oxygen evolution reaction†
Jingyuan
Zhang
a,
Zhen
Ma
ab,
Lanqi
Wang
a,
Hui
Ni
a,
Jianing
Yu
a and
Bin
Zhao
 *a
*a
aSchool of Materials and Chemistry, University of Shanghai for Science and Technology, Shanghai 200093, China. E-mail: zhaobin@usst.edu.cn
bBusiness development, Edwards Limited, Shanghai, China
First published on 7th May 2024
Abstract
Plasma treatment as an effective strategy can simultaneously achieve surface modification and heteroatom doping. Here, an N/P-doped NiFeV oxide nanosheet catalyst (N/P-NiFeVO) constructed by Ar/PH3 plasma treatment is used to drive the oxygen evolution reaction (OER). The introduction of V species leads to the formation of an ultrathin ordered nanostructure and exposure of more active sites. Compared to the 2D NiFeV LDH, the prepared N/P-NiFeVO by plasma treatment possesses multiple-valence Fe, V and Ni species, which regulate the intrinsic electronic structure and enable a superior catalytic activity for the OER in alkaline media. Specifically, the N/P-NiFeVO only require an overpotential of 273 mV to drive the current density of 100 mA cm−2. What's more, the electrode can maintain a stable current density in a long-term oxygen evolution reaction (∼120 h) under alkaline conditions. This work provides new insight for the rational design of mixed metal oxides for OER electrocatalysts.
Introduction
Hydrogen is considered to be a clean energy source with high energy density that can solve the problem of depletion of fossil resources and rapid consumption of energy.1–4 Electrochemical water splitting is considered to be one of the most efficient and sustainable strategies to produce hydrogen.5,6 Unfortunately, the sluggish kinetics during the OER process seriously affects the energy conversion efficiency.7,8 Noble metal Ru-based materials with best performance suffer from high cost and poor durability, which hinders development of renewable energy technologies.9,10 Thus, it is of great importance to develop OER electrocatalysts with low cost and high activity.11,12In the last few decades, a great variety of transition metal-based catalysts with low cost and high activities have been widely investigated, especially layered double hydroxides (LDHs).13–16 These materials are distinct 2D layer materials with excellent physicochemical characteristics, which makes it appropriate to design various hybrid materials with ion doping to improve the electrochemical activities. However, the OER activity of LDHs is limited by the low number of active sites at the edges and weak conductivity. Plasma treatment has been considered an efficient strategy to accelerate the OER process.17–19 It is found that surface etching and heteroatom doping can be achieved simultaneously under certain conditions, which can not only increase the active surface area, but also regulate the energy barrier during the OER process. Wang et al. reported a CoFe LDH and Co3O4 nanosheets with oxygen vacancies, which were created using plasma in Ar/water vapour. It is observed that the CoFe LDH and Co3O4 nanosheet catalysts exhibit lower overpotential values, meanwhile the Tafel value is only 68 mV dec−1.20 These results provide strong explanation for the successful improvement of OER performance. Liang et al. fabricated NiCoP nanosheets through Ar/PH3 plasma.21 The prepared nanosheets showed a lower potential value of 273 mV at a current density of 100 mA cm−2.
Herein, a novel approach is designed to fabricate an N/P-decorated Ni–Fe–V oxide electrocatalyst (N/P-NiFeVO). Facile preparation was realized by plasma treatment, which provided a greatly distinctive nanosheet structure, which was obtained from NiFeV-LDH precursors. The obtained NiFeV LDH with plasma treatment shows N/P doping and the formation of oxygen vacancies, which can largely enhance the electrochemical performance by providing a larger electrochemically active surface area and increased active sites. As an OER electrode, N/P-NiFeVO exhibited a low overpotential (273 mV at 100 mA cm−2) and an ultra-small Tafel value (34 mV dec−1) in 1 M KOH aqueous electrolyte. And it showed a stable catalytic activity with a high current density of 100 mA cm−2 for 120 h. This work sheds light on the preparation of high efficiency electrocatalysts.
Results and discussion
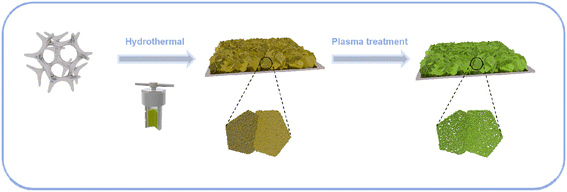
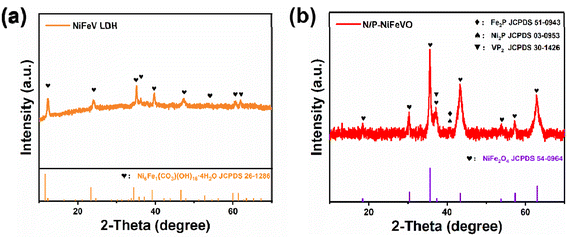
Scheme 1 is a schematic diagram of the synthesis procedure of N/P-NiFeVO. A one-step hydrothermal method is first carried out to synthesize a V-doped NiFe LDH on Ni foam (denoted as NiFeV LDH). Then, a nanostructure consisting of N/P-NiFeVO nanosheets with rich O vacancies is constructed via the following N2/PH3 plasma reduction step. As revealed in Fig. S2,† an optimum hydrothermal reaction time can result in the best OER performance. The NiFeV LDH-3 h precursor presents a smaller overpotential, lower Tafel slope and optimum charge transfer resistance than the other as-prepared samples. Consequently, the precursor prepared through a hydrothermal reaction of 3 h was chosen as the optimum sample.X-ray diffraction (XRD) spectroscopy was then employed to investigate the phase structure of the NiFeV LDH precursor and N/P-NiFeVO. The diffraction peaks in Fig. 1a can be well assigned to the NiFe LDH (JCPDS no. 26-1286). After N2 plasma treatment, all peaks in Fig. 1b from the NiFeV LDH vanish and the new diffraction peaks can be ascribed to the (111), (220), (311), (222), (400), (422), (511) and (440) planes of cubic NiFe2O4 (JCPDS no. 54-0964), demonstrating successful conversion from the LDH to the NiFe2O4 phase. The peaks appearing at 36.9° can be attributed to the (111) plane of VP2 (JCPDS: 30-1426). Additionally, the additional peak appearing at 40.7° can be attributed to the (111) plane of Fe2P (JCPDS: 51-0943) and Ni2P (JCPDS: 03-0953).
The initial NiFeV LDH and N/P-NiFeVO nanosheets mentioned above were synthesized and the surface morphology was characterized by scanning electron microscopy (SEM) as shown in Fig. 2a and b and transmission electron microscopy (TEM) as shown in Fig. 2c–g. The N/P-NiFeVO sample shows a similar nanosheet morphology, and plasma etching was employed to introduce more oxygen vacancies into the precursor. The TEM images revealed a relatively rough surface with small irregular pores on the ultrathin nanosheets, relative to the smooth surface of the pristine NiFeV LDH. The high-resolution TEM (HRTEM, Fig. 2d–g) images indicate the lattice fringe spacing of 0.15 nm and 0.25 nm consistent with the (440) and (311) planes characteristic of NiFe2O4 nanosheets. However, the 0.22 nm and 0.24 nm lattice fringes at the edges are attributed to the (111) and (111) planes of Ni2P/Fe2P (Fig. 2f and g), implying that the plasma etching preserves the P species. Furthermore, the selected area electron diffraction (SAED) pattern of N/P-NiFeVO can be well indexed to all the planes of Ni2P and Fe2P (Fig. 2h). The energy-dispersive X-ray spectroscopy (EDX) mapping images of N/P-NiFeVO show an atomic-level distribution of Ni, Fe, V, O, N and P in the whole nanosheet, manifesting the successful fabrication of N/P-NiFeVO.
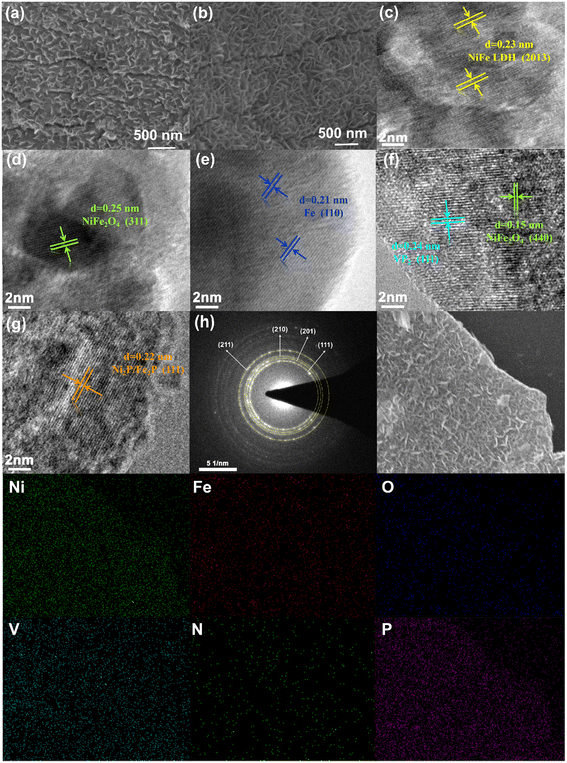 | ||
| Fig. 2 SEM (a) and HRTEM (c) images of the NiFeV LDH; (b) SEM and HRTEM (d–g) images of N/P-NiFeVO; (h) SAED pattern and the corresponding EDS elemental mapping of N/P-NiFeVO. | ||
X-ray photoelectron spectroscopy (XPS) was performed to further investigate the chemical environment and valence states of the NiFeV LDH and N/P-NiFeVO. For the NiFeV LDH, the XPS spectra of Ni 2p can be fitted with two distinct doublets (2p3/2 and 2p1/2). The peaks at 852.3 and 869.2 eV can be ascribed to the metal Ni, while the peaks at about 855.6 and 873.7 eV correspond to Ni2+.22,23 Regarding N/P-NiFeVO, the characteristic peaks at 856 and 870.3 eV in the Ni 2p spectrum indicate the presence of Ni+,24 as shown in Fig. 3a. In addition, after plasma treatment, the appearance of Fe2+ and Fe0 peaks confirms the partial reduction of the sample by PH3 plasma25 (Fig. 3b). In Fig. 3c, in comparison with the NiFeV LDH, the binding energy of V 2p of N/P-NiFeVO shifts to a lower binding energy, and the content of V3+ notably increases, which results from the reduction effect of PH3 plasma.26 In Fig. 3d, the peaks at 529.8, 531.3 and 532.4 eV are assigned to the M–O, oxygen vacancies and oxygen of adsorbed water, respectively.27 The intensities of oxygen vacancies and M–O of N/P-NiFeVO are enhanced compared to those of the NiFe LDH, indicating that the plasma etching employed introduced more oxygen vacancies into N/P-NiFeVO. The spectra of N 1s in Fig. 3e reveal the information about the valence states and chemical compositions. The N 1s spectra can be fitted with three peaks at 397.3, 398.8 and 401.1 eV, which are ascribed to V–N, Ni (Fe)–N, and N–O, respectively.28,29 In the P 2p spectrum (Fig. 3f), the peaks at 129.1 and 129.9 eV correspond to P 2p3/2 and P 2p1/2 of metal phosphide, and the peak at 133.4 eV is associated with P–O.30
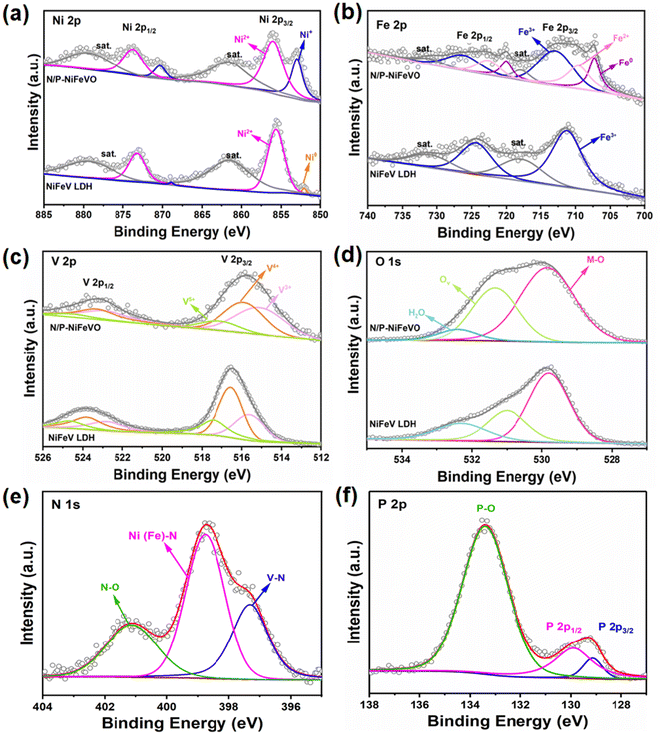 | ||
| Fig. 3 High-resolution XPS spectra of N/P-NiFeVO and NiFeV LDH: (a) Ni 2p, (b) Fe 2p, (c) V 2p, (d) O 1s, (e) N 1s and (f) P 2p. | ||
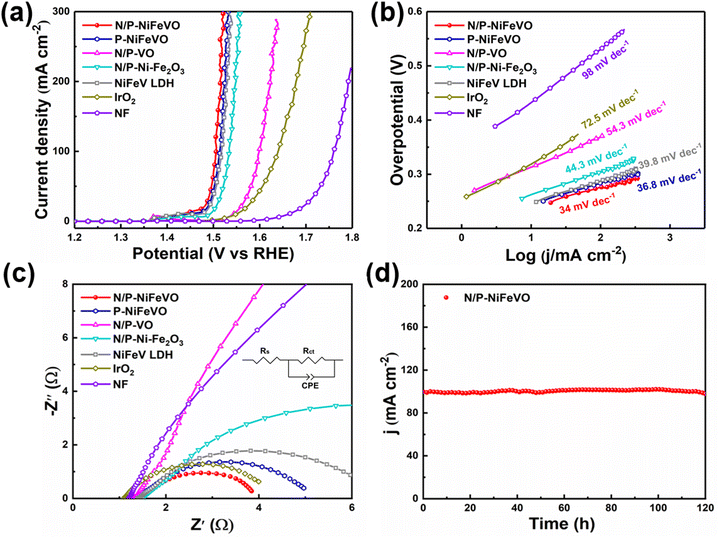
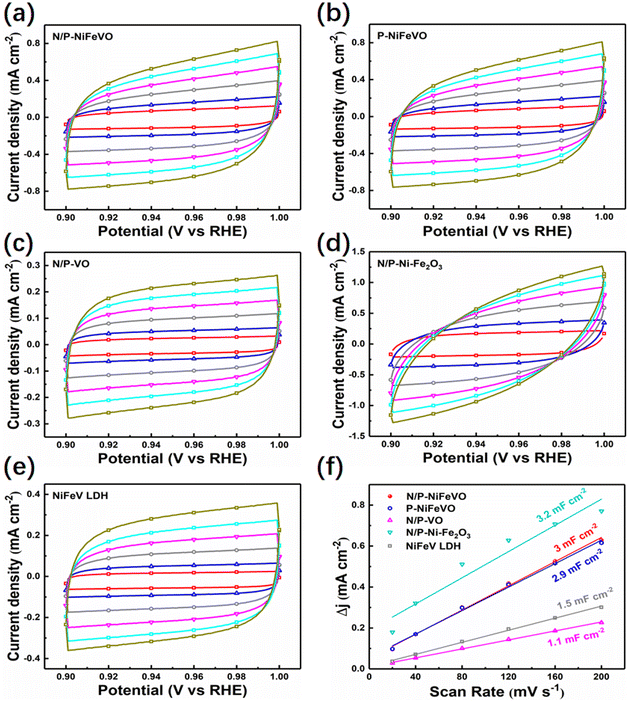
Subsequently, the OER performances of N/P-NiFeVO and derived samples were evaluated in 1 M KOH aqueous electrolyte using a standard three-electrode configuration (Fig. 4a). All potentials were iR-compensated and converted to a reversible hydrogen electrode (RHE) scale. As expected, compared with P-NiFeVO, N/P-VO, N/P–Ni Fe2O3, NiFeV LDH and IrO2, N/P-NiFeVO exhibited the lowest overpotential at the same current density (273 mV at 100 mA cm−2) and an ultra-small Tafel value (34 mV dec−1), suggesting that the plasma etched electrocatalyst with ultrathin nanosheets can serve as a good electrocatalytically active species for the OER (Fig. 4b). Noteworthily, such a low overpotential is superior to those of many OER heterostructured catalysts (Table S1†). In addition, the electrochemically active area (ECSA) was calculated from cyclic voltammetry (Fig. 5) at different scan rates from 20 to 120 mV s−1 within the non-faradaic potential range. It is clear that the ECSA of N/P-NiFeVO nanosheets with plasma treatment is larger than that of the NiFeV LDH material. In other words, the surface reconstruction by plasma treatment does not compromise the high specific surface of the original catalyst. As shown in Fig. 5f, the Cdl of N/P-NiFeVO is 3 mF cm−2, noticeably larger than that of the NiFeV LDH and its derivatives (1.5 mF cm−2), which indicates that more active sites were generated. To gain more insights into the intrinsic activity for the OER, the LSV polarization curves were normalized with ECSA. As depicted in Fig. S4,† N/P-NiFeVO possesses superior intrinsic catalytic activity compared to the other catalysts.31,32 It can be inferred that N/P-NiFeVO constructed by plasma treatment has more exposed active sites, which enable its remarkable catalytic activities.33,34
At the same time, its stability is also outstanding, showing good stability up to 120 h with little change at a constant current density of 100 mA cm−2 (Fig. 4d). Further SEM images of the post-OER catalyst show a rougher structure with irregular clusters on nanosheets (Fig. S5†). In the surface oxidation under anodic voltage, the morphology of the catalyst was largely retained compared to that of the as-prepared sample. After the OER test, the high-resolution XPS spectra of Ni 2p (Fig. S6a†) show two peaks ascribed to Ni2+, while the Ni0 peak completely disappeared. In addition, the only remaining Fe3+ peak confirms the complete oxidation of Fe0 and Fe2+ (Fig. S6b†). In Fig. S6c,† the peaks at 530.3, 531.3 and 532.6 eV are assigned to the M–O, oxygen vacancies and oxygen of adsorbed water, respectively.35,36 Fig. S6d† shows peaks at 515.4, 516.2 and 517.1 eV, which are assigned to V3+, V4+and V5+. Compared to those of N/P-NiFeVO, the intensities of V–N, Ni (Fe)–N and N–O of the post-OER catalyst weakened and the P 2p peak completely disappeared, which is due to the intense oxidation process (Fig. S6e and f†).
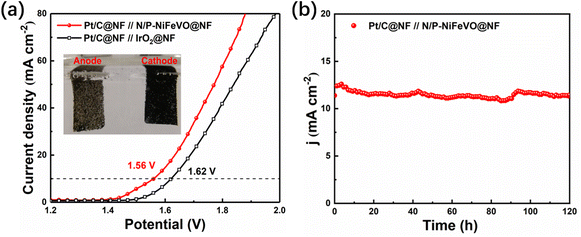
To evaluate the durability of N/P-NiFeVO in alkaline media, we assembled a two-electrode system simulating a water splitting cell using Pt/C as a hydrogen evolution catalyst and N/P-NiFeVO as an oxygen evolution catalyst. As shown in Fig. 6a, the cell voltages in alkaline media of Pt/C∥N/P-NiFeVO and Pt/C∥IrO2 are 1.56 V@10 mA cm−2 and 1.62 V@10 mA cm−2, respectively. Moreover, for the Pt/C∥N/P-NiFeVO, the cell voltage can remain at a similar level even after continuous working for 120 h. Such a performance for water electrolysis is better than that of most recently reported NiFe-based electrocatalysts (Table S2†). Overall, these results clearly indicate that N/P-NiFeVO is indeed a competent and persistent OER catalyst.
Conclusion
In summary, a well-aligned N/P-doped NiFeVO oxide nanosheet catalyst was prepared by Ar/PH3 plasma treatment of the NiFe LDH. Plasma treatment not only leads to the transformation of the NiFe LDH to NiFeVO nanosheets, but also induces the formation of oxygen vacancies. Accordingly, the N/P-doped NiFeVO nanosheets with a high specific surface area and plentiful pores provide unobstructed channels for electrolyte ion diffusion and boosted charge transfer kinetics. These advantages endow the N/P-doped NiFeVO electrode with outstanding electrochemical properties. This work shows that tuning the synthesis conditions and composition of mixed metal oxides is an effective strategy to enhance the catalytic performance for the OER.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
Financial support from the Natural Science Foundation of Shanghai (21ZR1445700) and the Science and Technology Commission of Shanghai Municipality (23440790400) is gratefully acknowledged.References
- L. Xu, S. Wu, X. He, H. Wang, D. Deng, J. Wu and H. Li, Chem. Eng. J., 2022, 437, 135291 CrossRef CAS.
- P. Erickson, M. Lazarus and G. Piggot, Nat. Clim. Change, 2018, 8, 1037–1043 CrossRef CAS.
- F. P. Perera, Environ. Health Perspect., 2017, 125, 141–148 CrossRef CAS PubMed.
- R. Gao and D. P. Yan, Adv. Energy Mater., 2020, 10, 1900954 CrossRef CAS.
- T. R. Cook, D. K. Dogutan, S. Y. Reece, Y. Surendranath, T. S. Teets and D. G. Nocera, Chem. Rev., 2010, 110, 6474–6502 CrossRef CAS PubMed.
- R. Gao, H. Zhang and D. P. Yan, Nano Energy, 2017, 31, 90–95 CrossRef CAS.
- N. T. Suen, S. F. Hung, Q. Quan, N. Zhang, Y. J. Xu and H. M. Chen, Chem. Soc. Rev., 2017, 46, 337–365 RSC.
- M. Arif, G. Yasin, M. Shakeel, M. A. Mushtaq, W. Ye, X. Y. Fang, S. F. Ji and D. P. Yan, Mater. Chem. Front., 2019, 3, 520–531 RSC.
- D. Chen, R. Lu, Z. Pu, J. Zhu, H. W. Li, F. Liu, S. Hu, X. Luo, J. Wu, Y. Zhao and S. Mu, Appl. Catal., B, 2020, 279, 119396 CrossRef CAS.
- Y. Lee, J. Suntivich, K. J. May, E. E. Perry and Y. Shao-Horn, J. Phys. Chem. Lett., 2012, 3, 399–404 CrossRef CAS PubMed.
- Q. Q. Ji, Y. Kong, H. Tan, H. L. Duan, N. Li, B. Tang, Y. Wang, S. H. Feng, L. Y. Lv, C. Wang, F. C. Hu, W. H. Zhang, L. Cai and W. S. Yan, ACS Catal., 2022, 12, 4318–4326 CrossRef CAS.
- S. Jin, ACS Energy Lett., 2017, 2, 1937 CrossRef CAS.
- G. F. Chen, T. Y. Ma, Z. Q. Liu, N. Li, Y. Z. Su, K. Davey and S. Z. Qiao, Adv. Funct. Mater., 2016, 26, 3314–3323 CrossRef CAS.
- J. Yu, T. A. Le, N. Q. Tran and H. Lee, Chem. – Eur. J., 2020, 26, 6423–6436 CrossRef CAS PubMed.
- C. Hu, L. Zhang and J. Gong, Energy Environ. Sci., 2019, 12, 2620–2645 RSC.
- S. Dresp, F. Dionigi, M. Klingenhof, T. Merzdorf, H. Schmies, J. Drnec, A. Poulain and P. Strasser, ACS Catal., 2021, 11, 6800–6809 CrossRef CAS.
- F. Song and X. Hu, Exfoliation of layered double hydroxides for enhanced oxygen evolution catalysis, Nat. Commun., 2014, 5, 4477 CrossRef CAS PubMed.
- L. Ma, I. Jia, X. Guo and L. Xiang, Chin. J. Catal., 2014, 35, 108–119 CrossRef CAS.
- A. U. Haq, S. Askari, A. McLister, S. Rawlinson, J. Davis, S. Chakrabarti, V. Svrcek, P. Maguire, P. Papakonstantinou and D. Mariotti, Nat. Commun., 2019, 10, 1–8 CrossRef PubMed.
- R. Liu, Y. Wang, D. Liu, Y. Zou and S. Wang, Adv. Mater., 2017, 29, 1–7 Search PubMed.
- H. Liang, A. N. Gandi, D. H. Anjum, X. Wang, U. Schwingenschlögl and H. N. Alshareef, Nano Lett., 2016, 16, 7718–7725 CrossRef CAS PubMed.
- A. L. Wang, H. Xu and G. R. Li, ACS Energy Lett., 2016, 1, 445–453 CrossRef CAS.
- H. J. Zhang, X. P. Li, A. Hähnel, V. Naumann, C. Lin, S. Azimi, S. L. Schweizer, A. W. Maijenburg and R. B. Wehrspohn, Adv. Funct. Mater., 2018, 28, 1706847 CrossRef.
- P. Zhou, D. N. Xing, Y. Y. Liu, Z. Y. Wang, P. Wang, Z. K. Zheng, X. Y. Qin, X. Y. Zhang, Y. Dai and B. B. Huang, J. Mater. Chem. A, 2019, 7, 5513–5521 RSC.
- X. L. Liu, X. S. Lv, P. Wang, Q. Q. Zhang, B. B. Huang, Z. Y. Wang, Y. Y. Liu, Z. K. Zheng and Y. Dai, Electrochim. Acta, 2020, 333, 135488 CrossRef CAS.
- Y. M. Hu, Z. L. Wang, W. J. Liu, L. Xu, M. L. Guan, Y. P. Huang, Y. Zhao, J. Bao and H. M. Li, ACS Sustainable Chem. Eng., 2019, 7, 16828–16834 CrossRef CAS.
- H. H. Lv, X. B. Zhang, F. M. Wang, G. J. Lv, T. T. Yu, M. L. Lv, J. W. Wang, Y. Zhai and J. Q. Hu, J. Mater. Chem. A, 2020, 8, 14287–14298 RSC.
- M. L. Yan, K. Mao, P. X. Cui, C. Chen, J. Zhao, X. Z. Wang, L. J. Yang, H. Yang, Q. Wu and Z. Hu, Nano Res., 2020, 13, 328–334 CrossRef CAS.
- S. S. Tan, Y. H. Dai, Y. L. Jiang, Q. L. Wei, G. B. Zhang, F. Y. Xiong, X. Q. Zhu, Z. Y. Hu, L. Zhou, Y. C. Jin, K. Kanamura, Q. Y. An and L. Q. Mai, Adv. Funct. Mater., 2020, 2008034 Search PubMed.
- M. J. Guo, S. Y. Song, S. S. Zhang, Y. Yan, K. Zhan, J. H. Yang and B. Zhao, ACS Sustainable Chem. Eng., 2020, 8, 7436–7444 CrossRef CAS.
- N. Li, L. Cai, C. Wang, Y. Lin, J. Z. Huang, H. Y. Sheng, H. B. Pan, W. Zhang, Q. Q. Ji, H. L. Duan, W. Hu, W. H. Zhang, F. C. Hu, H. Tan, Z. H. Sun, B. Song, S. Jin and W. S. Yan, J. Am. Chem. Soc., 2021, 143, 18001–18009 CrossRef CAS PubMed.
- N. Li, L. Cai, G. P. Gao, Y. Lin, C. Wang, H. J. Liu, Y. Y. Liu, H. L. Duan, Q. Q. Ji, W. Hu, H. Tan, Z. M. Qi, L. W. Wang and W. S. Yan, Nano Lett., 2022, 22, 6988–6996 CrossRef CAS PubMed.
- Y. Yan, C. Liu, H. Jian, X. Cheng, T. Hu, D. Wang, L. Shang, G. Chen, P. Schaaf, X. Wang, E. Kan and T. Zhang, Adv. Funct. Mater., 2021, 31, 2009610 CrossRef CAS.
- Z. Wang, B. Xiao, Z. Lin, Y. Xu, Y. Lin, F. Meng, Q. Zhang, L. Gu, B. Fang, S. Guo and W. Zhong, Angew. Chem., Int. Ed., 2021, 60, 23388–23393 CrossRef CAS PubMed.
- Z. Xu, Y. Ying, G. Zhang, K. Li, Y. Liu, N. Fu, X. Guo, F. Yu and H. Huang, J. Mater. Chem. A, 2020, 8, 26130 RSC.
- S. Klaus, Y. Cai, M. W. Louie, L. Trotochaud and A. T. Bell, J. Phys. Chem. C, 2015, 119, 7243 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4dt00943f |
| This journal is © The Royal Society of Chemistry 2024 |