Open Access Article
Open Access ArticleStepwise construction of coordinative linkages and dynamic covalent linkages for a porous metal–organic framework†
Shuyin
Peng
,
Yuqian
Sun
,
Qingqing
Li
,
Zhongwen
Jiang
,
Yin
Rao
,
Yichen
Wu
and
Qiaowei
Li
 *
*
Department of Chemistry, Collaborative Innovation Center of Chemistry for Energy Materials, and Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Fudan University, Shanghai 200433, P. R. China. E-mail: qwli@fudan.edu.cn
First published on 6th January 2024
Abstract
A cyclic trinuclear complex is synthesized from AgI and 1H-pyrazole-4-carbaldehyde. Reticulation of the complex with 1,3,5-tris(4-aminophenyl)benzene through Schiff-base reaction affords a porous FDM-72 framework. Amine choice is systematically investigated as it may initiate metal reduction. This study proposes a new route and its amine selection criterion to synthesize Ag-based frameworks.
Metal–organic frameworks (MOFs)1 are a class of crystalline porous materials typically constructed from inorganic secondary building units (SBUs)2 and organic linkers. Coordination linkages between metal ions and organic binding groups (carboxylates, azolates, etc.) are the main driving force that bring different constituents together and lock each other into well-defined frameworks. Recently, as an alternative route to synthesize MOFs, discrete coordination complexes with specific functional groups are being employed to react with organic units using dynamic covalent chemistry to achieve extended structures.3 For example, [Ti6O6(COO)6] clusters,4 [Cr3O(COO)6] clusters,5 [Zn4(PO3)4] clusters,6 and even polyoxometalates7 with –NH2/–CHO functionalities have been reticulated with organic aldehyde/amine through Schiff-base reaction. As a juncture of MOF and covalent organic framework (COF) chemistry,8 the newly developed synthetic route involves the construction of both coordination linkages and dynamic covalent linkages in one framework.
In 2021, Li and our group reported the use of Cu3(PyCA)3 (HPyCA = 1H-pyrazole-4-carbaldehyde) as the basic modules for MOF construction through dynamic covalent chemistry.9 The synthetic approaches have been practiced to build various reticular materials9,10 targeting small molecule catalysis and photocatalytic CO2 conversion applications, thanks to their abundant redox-active sites and periodic π–π conjugated system. However, applying the same stepwise synthetic strategy to frameworks based on other d10 metals, such as Ag, is less investigated. (i) For the coordination linkage construction, facile preparation and isolation of molecular Ag3(PyCA)3 has not been reported yet, and its crystal structure is desired for precise modelling of the corresponding extended structures. (ii) For the dynamic covalent linkage construction, AgI is more oxidative than CuI, and their compatibility with various amines featuring different reductive ability remains unexplored.
Herein, we report the successful synthesis of Ag3(PyCA)3 molecular single crystals through Ag–pyrazolate coordination. The triangular complex could serve as a 3-connected node when reacting with 1,3,5-tris(4-aminophenyl)benzene (TAPB) through Schiff-base reaction. With the help of stepwise coordination linkage and dynamic covalent linkage, AgI, PyCA, and TAPB are successfully reticulated into a two-dimensional (2D) honeycomb structure with layers packed in eclipsed fashion. Furthermore, the metal/amine compatibility investigation reveals that, while both p-phenylenediamine (PA) and TAPB are compatible with Cu3(PyCA)3, PA may reduce Ag3(PyCA)3 and complicate the framework construction. This study proposes not only a new route to synthesize Ag-based frameworks, but also a criterion for their building unit selection.
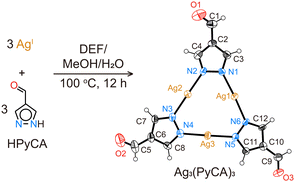
Through a solvothermal reaction of AgNO3 and HPyCA in a mixture of N,N-diethylformamide (DEF), methanol, and H2O at 100 °C for 12 h, Ag3(PyCA)3 crystals in needle shape are synthesized (Fig. 1, see the ESI,† for details). Single-crystal X-ray diffraction (SXRD) reveals that each AgI is linearly coordinated with two N from two (PyCA)−, and three AgI form a trinuclear triangular complex with three (PyCA)−. The complex with three –CHO groups is co-planar. In each unit cell, 16 Ag3(PyCA)3 molecules are arranged in a 4 × 4 fashion (Fig. S5a, ESI†). Slight rotation and tilting between neighbouring complexes results in a unit cell with long a and b cell parameters (Table S1, ESI†). π–π stacking between the complexes is evident, as the intermolecular distance is only 3.0 Å (Fig. S5b, ESI†).
It should be noted that AgI is likely to be reduced to Ag during the complex synthesis, thus the obtained product is often a mixture of colorless Ag3(PyCA)3 crystals and black Ag particles (Fig. S1, ESI†). However, the Ag particles can be easily removed by dissolving Ag3(PyCA)3 in DMSO, followed by filtration and re-precipitation in methanol (Fig. S2, ESI†). Ag3(PyCA)3 is isostructural with the previously reported Cu3(PyCA)3·H2O and Ag3(Me2PyBCA)3 (Me2PyBCA = 4-(3,5-dimethyl-pyrazol-4-yl)benzaldehyde).9b,11 With three –CHO in the corners, the triangular Ag3(PyCA)3 could serve as a building unit to further build extended structures using dynamic covalent chemistry.
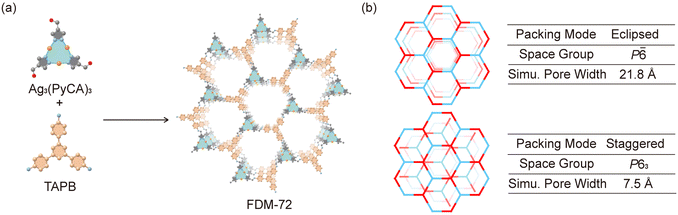
A crystalline material named FDM-72 (FDM = FuDan Materials) is obtained after heating Ag3(PyCA)3 in a TAPB solution in mesitylene, dioxane, and aqueous CH3COOH at 50 °C for 72 h (Fig. 2a). Compared with the Fourier transform infrared (FT-IR) spectra of Ag3(PyCA)3 and TAPB, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching (1660 cm−1) and N–H stretching (3424 and 3346 cm−1) are not observed in FDM-72 (Fig. S3, ESI†). Furthermore, a new vibration at 1617 cm−1 in FDM-72 confirms the C
O stretching (1660 cm−1) and N–H stretching (3424 and 3346 cm−1) are not observed in FDM-72 (Fig. S3, ESI†). Furthermore, a new vibration at 1617 cm−1 in FDM-72 confirms the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond formation, suggesting a Schiff-base reaction between Ag3(PyCA)3 and TAPB.
N bond formation, suggesting a Schiff-base reaction between Ag3(PyCA)3 and TAPB.
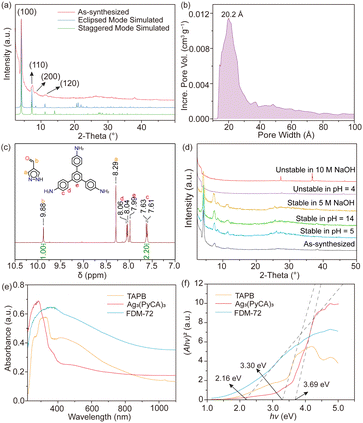
FDM-72 shows relatively strong low angle peaks (Fig. 3a) in powder X-ray diffraction (PXRD); however, determining its crystal structure using SXRD is not possible due to its small crystal size and inherent structural disorder. In addition, the PXRD with slight amorphization and weak diffraction at high angles is not enough for a reasonable Rietveld refinement.10d,g Since both Ag3(PyCA)3 and TAPB show C3 symmetry, it is rational to build an FDM-72 structure model as 2D honeycomb layers,12 in which the tritopic Ag3(PyCA)3 and TAPB nodes are arranged alternatively within the layers (Fig. 2a). We modelled the two most possible stacking modes of the layers after applying geometry optimization of the constituents, and they are (i) eclipsed mode with AA packing of layers along the c axis in the P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) space group, and (ii) the staggered mode with AB packing in space group P63 (Fig. 2b). Pawley refinement13 is further performed based on the two structure models (see the ESI†). Specifically, for the eclipsed model, its unit cell parameters are refined to be a = 24.0661 Å, c = 4.2981 Å (Table S2 and Fig. S6, ESI†). On the other hand, for the staggered model, the parameters after refinement come to a = 23.7252 Å, c = 6.5299 Å (Table S3 and Fig. S7, ESI†). The experimental PXRD peaks at 2θ = 4.3, 7.5, 8.6 and 11.5° could be assigned to (100), (110), (200), and (120) planes in both models. However, the peaks at higher angles are relatively wide and weak (Fig. 3a), and unambiguous assignment to different planes in either model is not possible. Although the Pawley refinement shows marginally better agreement for the eclipsed model, determining the exact stacking mode of FDM-72 with high confidence needs additional experimental validation, such as its pore size distribution analysis.
space group, and (ii) the staggered mode with AB packing in space group P63 (Fig. 2b). Pawley refinement13 is further performed based on the two structure models (see the ESI†). Specifically, for the eclipsed model, its unit cell parameters are refined to be a = 24.0661 Å, c = 4.2981 Å (Table S2 and Fig. S6, ESI†). On the other hand, for the staggered model, the parameters after refinement come to a = 23.7252 Å, c = 6.5299 Å (Table S3 and Fig. S7, ESI†). The experimental PXRD peaks at 2θ = 4.3, 7.5, 8.6 and 11.5° could be assigned to (100), (110), (200), and (120) planes in both models. However, the peaks at higher angles are relatively wide and weak (Fig. 3a), and unambiguous assignment to different planes in either model is not possible. Although the Pawley refinement shows marginally better agreement for the eclipsed model, determining the exact stacking mode of FDM-72 with high confidence needs additional experimental validation, such as its pore size distribution analysis.
The N2 adsorption isotherm of FDM-72 is measured at 77 K after solvent exchange and sample activation. Moderate PXRD intensity still remains after the MOF is washed with N,N-dimethylformamide (DMF) (Fig. S8, ESI†), followed by supercritical CO2 drying and 100 °C heating in vacuo. The adsorption isotherm can be characterized as a type IV isotherm featuring a clear hysteresis loop (Fig. S9, ESI†). The Brunauer–Emmett–Teller surface area (ABET) of activated FDM-72 is calculated to be 395 m2 g−1, and its total pore volume is 0.448 cm3 g−1. We found that the experimental ABET is much lower than the simulated values based on the eclipsed (2118 m2 g−1) or staggered model (1088 m2 g−1) using Zeo++,14 indicating substantial inherent defects in FDM-72 and possible local structural collapse during the sample activation. Based on nonlocal density functional theory (NLDFT), FDM-72 features a wide pore size distribution between ∼13 and 40 Å, with the peak centre at 20 Å (Fig. 3b). Pores larger than 40 Å are also evident, further confirming macroscopic defects in the framework.
Although the experimental ABET is lower than the simulation, previous studies show that pore size is still a strong indicator of the layer packing mode.9b,15 Theoretically, FDM-72 with eclipsed packing would have mesopores with 21.8 Å in diameter, while only micropores (d = 7.5 Å) exist in the staggered model. Comparing the experimental with the simulated pore size, we conclude that the layers in FDM-72 are stacked in eclipsed mode. Overall, the Ag3-based FDM-72 is isostructural with the Cu3-based JNM-1,9a suggesting that the Ag/Cu–pyrazolate coordination linkage doesn’t interfere with the imine linkage formation, and a rational stepwise synthetic strategy could be developed for MOFs based on the trinuclear d10 metal complex.
To quantify the organic moieties in FDM-72, it is first digested by DCl and the obtained organic components are separated from AgCl precipitates for solution 1H NMR measurement. Both the Ag–pyrazolate coordination linkage and the covalent imine linkage are dissociated during the digestion. Peak integration concludes that the molar ratio between PyCA and TAPB is 3.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 in FDM-72 (Fig. 3c), which is slightly deviated from the theoretical value (3
1.1 in FDM-72 (Fig. 3c), which is slightly deviated from the theoretical value (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The observation suggests that PyCA vacancies are dominant in FDM-72.
1). The observation suggests that PyCA vacancies are dominant in FDM-72.
Scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX) confirm uniform element distribution in the crystalline material (Fig. S10, ESI†). X-Ray photoelectron spectroscopy (XPS) confirms that the oxidation state of Ag in FDM-72 is +1 (Fig. S11, ESI†). After the samples are kept in solutions with pH = 5–14, their PXRD patterns remain intact (Fig. 3d). Furthermore, FDM-72 still remains crystalline after it is soaked in NaOH (5 M) for 7 days, confirming the high chemical stability of both the Ag–N coordination and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N imine linkages. Further increasing the NaOH concentration to 10 M or lowering the acid pH value to 4 leads to the decomposition of the framework. The Ag3(PyCA)3 complex starts to decompose at 200 °C in thermogravimetric analysis (Fig. S12, ESI†). After the complexes are incorporated into the framework, FDM-72 is thermally stable up to 330 °C, indicating that the resulting scaffold is more resilient towards heat treatment after the formation of the imine linkage.
N imine linkages. Further increasing the NaOH concentration to 10 M or lowering the acid pH value to 4 leads to the decomposition of the framework. The Ag3(PyCA)3 complex starts to decompose at 200 °C in thermogravimetric analysis (Fig. S12, ESI†). After the complexes are incorporated into the framework, FDM-72 is thermally stable up to 330 °C, indicating that the resulting scaffold is more resilient towards heat treatment after the formation of the imine linkage.
Highly conjugated π-orbital arrays in FDM-72 would enhance charge delocalization, resulting in small band gaps that contribute to high photoenergy conversion performances. In the UV-vis diffuse reflectance spectrum, the absorbance of the deep brown FDM-72 in the visible range is greatly signified compared with those of Ag3(PyCA)3 and TAPB, with the absorption peak at 400 nm (Fig. 3e). Using the Kubelka–Munk function, the optical band gap (Eg) of Ag3(PyCA)3 and TAPB is calculated to be 3.69 and 3.30 eV, respectively (Fig. 3f). After they are reticulated into the 2D framework, the optical band gap of FDM-72 is only 2.16 eV, confirming that the imine linkages build up a highly conjugated π–π system.16 The electrochemical Mott–Schottky spectrum and valence band X-ray photoelectron spectrum (VB-XPS) of FDM-72 are further measured. Its conduction band position is estimated to be −0.38 V versus NHE (Fig. S13, ESI†), and the valence band edge is located at 1.73 eV (Fig. S14, ESI†). The calculated band gap (2.11 eV) based on the Mott–Schottky plots and the VB-XPS agrees with the optical band gap (2.16 eV).
AgI is known to be reduced easily even by a mild reducing agent, and a direct coordinative assembly between d10 metals (such as AgI and AuI) and organic linkers often encounters unavoidable Ag or Au particles as by-products.17 Separating the metal particles from the framework solids is not straightforward for most systems. In this work, we construct FDM-72 by connecting the coordination complex with tritopic amine through Schiff-base reaction. The molecular Ag3(PyCA)3 allows us to remove unwanted metal particles before the framework construction by simple dissolution and filtration, and thus the consequent FDM-72 is guaranteed to be phase pure. However, we are aware that different organic amines may have different redox potential, and the compatibility between the Ag3(PyCA)3 complex and different amines needs to be investigated to define the limit of the presented synthetic strategy.
We picked two isostructural trinuclear complexes based on two kinds of d10 metals (CuI and AgI) and two kinds of organic amines (TAPB and PA) for the compatibility study. Cu3(PyCA)3 is known to react with TAPB or PA to obtain JNM-19a and FDM-719b (Fig. 4a and b). No diffraction peak from Cu is observed in the product PXRD, confirming that neither amine could reduce CuI during the framework synthesis. Successful construction of FDM-72 proves that TAPB could not reduce AgI in Ag3(PyCA)3 to Ag (Fig. 4c). We now turn to the reaction between Ag3(PyCA)3 and PA to study the competition between Ag reduction and Schiff-base reaction in various MOF synthesis conditions.
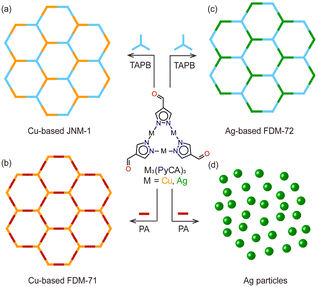 | ||
| Fig. 4 Framework construction based on M3(PyCA)3 (M = Cu, Ag) and organic amines (TAPB and PA). (a) Cu-based JNM-1,9a (b) Cu-based FDM-71,9b and (c) Ag-based FDM-72 (this work) can be successfully obtained; however, (d) PA could reduce AgI in the Ag3(PyCA)3 complex and only Ag particles are formed. | ||
Several attempts, such as mechanical grinding and solvothermal reactions in vials or Schlenk flasks, were applied to the reaction of Ag3(PyCA)3 and PA. However, no crystalline framework was formed and only Ag particles were detected in PXRD (Fig. 4d and Fig. S4, ESI†). The reductive ability of PA is strong enough to reduce AgI in the complex and thus destroys the complex, while that of TAPB is weaker and does not lead to Ag precipitates. In the case of CuI-based Cu3(PyCA)3, using either PA or TAPB is safe for framework construction. Further cyclic voltammetry (CV) of PA and TAPB (0.054 M) reveals their oxidation potential at 0.58 and 1.12 V (versus SHE) (Fig. S15, ESI†). Given the reference that standard electrode potentials (E°) of AgI(aq) + e− → Ag(s) and CuI(aq) + e− → Cu(s) are 0.80 and 0.52 V, respectively, it is reasonable that the AgI and PA combination undergoes redox reaction, thus interrupting the framework construction. Overall, we propose that the coordination linkage formation, the dynamic covalent linkage formation, and the redox potential compatibility between the constituents all need to be considered for functional framework synthesis.
In summary, we report the synthesis and structure of the cyclic Ag3(PyCA)3 complex based on the coordination between three linear AgI and three PyCA ligands. The complex with three –CHO groups can serve as a 3-connected node for reticular structure. Furthermore, the dynamic covalent linkage between the complex and TAPB gives rise to FDM-72 with honeycomb layers stacked in an eclipsed fashion. FDM-72 shows 1D channels with 2.0 nm diameter, and an optical band gap of only 2.16 eV. The oxidative potential compatibility between the amine and the metal needs to be considered for the stepwise linkage construction, since the reductive amine may reduce the metal and thus interrupt the corresponding framework synthesis.
The authors are thankful for the financial support from the National Natural Science Foundation of China (Grants 21922103, 21961132003, 22088101).
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) O. M. Yaghi, G. Li and H. Li, Nature, 1995, 378, 703–706 CrossRef CAS; (b) S. Kitagawa, R. Kitaura and S.-I. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334–2375 CrossRef CAS PubMed; (c) G. Férey, Chem. Soc. Rev., 2008, 37, 191–214 RSC; (d) J.-P. Zhang, Y.-B. Zhang, J.-B. Lin and X.-M. Chen, Chem. Rev., 2012, 112, 1001–1033 CrossRef CAS PubMed; (e) H. Furukawa, K. E. Cordova, M. O’Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed; (f) S. Yuan, L. Feng, K. Wang, J. Pang, M. Bosch, C. Lollar, Y. Sun, J. Qin, X. Yang, P. Zhang, Q. Wang, L. Zou, Y. Zhang, L. Zhang, Y. Fang, J. Li and H.-C. Zhou, Adv. Mater., 2018, 30, 1704303 CrossRef PubMed.
- M. J. Kalmutzki, N. Hanikel and O. M. Yaghi, Sci. Adv., 2018, 4, eaat9180 CrossRef CAS PubMed.
- (a) J. Dong, X. Han, Y. Liu, H. Li and Y. Cui, Angew. Chem., Int. Ed., 2020, 59, 13722–13733 CrossRef CAS PubMed; (b) Y. Li, M. Karimi, Y.-N. Gong, N. Dai, V. Safarifard and H.-L. Jiang, Matter, 2021, 4, 2230–2265 CrossRef CAS.
- (a) H. L. Nguyen, F. Gándara, H. Furukawa, T. L. H. Doan, K. E. Cordova and O. M. Yaghi, J. Am. Chem. Soc., 2016, 138, 4330–4333 CrossRef CAS PubMed; (b) H. L. Nguyen, T. T. Vu, D. Le, T. L. H. Doan, V. Q. Nguyen and N. T. S. Phan, ACS Catal., 2017, 7, 338–342 CrossRef CAS.
- S. K. Elsaidi, M. H. Mohamed, J. S. Loring, B. P. McGrail and P. K. Thallapally, ACS Appl. Mater. Interfaces, 2016, 8, 28424–28427 CrossRef CAS PubMed.
- R. Jangir, A. C. Kalita, D. Kaleeswaran, S. K. Gupta and R. Murugavel, Chem. – Eur. J., 2018, 24, 6178–6190 CrossRef CAS PubMed.
- (a) W. Xu, X. Pei, C. S. Diercks, H. Lyu, Z. Ji and O. M. Yaghi, J. Am. Chem. Soc., 2019, 141, 17522–17526 CrossRef CAS PubMed; (b) R. Ma, N. Liu, T.-T. Lin, T. Zhao, S.-L. Huang and G.-Y. Yang, J. Mater. Chem. A, 2020, 8, 8548–8553 RSC.
- (a) A. P. Côté, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166–1170 CrossRef PubMed; (b) C. S. Diercks and O. M. Yaghi, Science, 2017, 355, eaal1585 CrossRef PubMed; (c) K. Geng, T. He, R. Liu, S. Dalapati, K. T. Tan, Z. Li, S. Tao, Y. Gong, Q. Jiang and D. Jiang, Chem. Rev., 2020, 120, 8814–8933 CrossRef CAS PubMed.
- (a) R.-J. Wei, H.-G. Zhou, Z.-Y. Zhang, G.-H. Ning and D. Li, CCS Chem., 2021, 3, 2045–2053 CrossRef CAS; (b) X. Li, J. Wang, F. Xue, Y. Wu, H. Xu, T. Yi and Q. Li, Angew. Chem., Int. Ed., 2021, 60, 2534–2540 CrossRef CAS PubMed.
- (a) H.-G. Zhou, R.-Q. Xia, J. Zheng, D. Yuan, G.-H. Ning and D. Li, Chem. Sci., 2021, 12, 6280–6286 RSC; (b) J. Zhou, J. Li, L. Kan, L. Zhang, Q. Huang, Y. Yan, Y. Chen, J. Liu, S.-L. Li and Y.-Q. Lan, Nat. Commun., 2022, 13, 4681 CrossRef CAS PubMed; (c) R.-J. Wei, P.-Y. You, H. Duan, M. Xie, R.-Q. Xia, X. Chen, X. Zhao, G.-H. Ning, A. I. Cooper and D. Li, J. Am. Chem. Soc., 2022, 144, 17487–17495 CrossRef CAS PubMed; (d) X. Wang, X. Ding, Y. Jin, D. Qi, H. Wang, Y. Han, T. Wang and J. Jiang, Angew. Chem., Int. Ed., 2023, 62, e202302808 CrossRef CAS PubMed; (e) J.-N. Chang, J.-W. Shi, Q. Li, S. Li, Y.-R. Wang, Y. Chen, F. Yu, S.-L. Li and Y.-Q. Lan, Angew. Chem., Int. Ed., 2023, 62, e202303606 CrossRef PubMed; (f) X.-C. Lin, Y.-M. Wang, X. Chen, P.-Y. You, K.-M. Mo, G.-H. Ning and D. Li, Angew. Chem., Int. Ed., 2023, 62, e202306497 CrossRef CAS PubMed; (g) M. Zhang, P. Huang, J.-P. Liao, M.-Y. Yang, S.-B. Zhang, Y.-F. Liu, M. Lu, S.-L. Li, Y.-P. Cai and Y.-Q. Lan, Angew. Chem., Int. Ed., 2023, 62, e202311999 CrossRef CAS PubMed; (h) J.-Y. Song, X. Chen, Y.-M. Wang, X. Luo, T.-E. Zhang, G.-H. Ning and D. Li, Chem. – Asian J., 2023, 18, e202300857 CrossRef CAS PubMed.
- H. Li, J. Luo, Z.-Y. Zhang, R.-J. Wei, M. Xie, Y.-L. Huang, G.-H. Ning and D. Li, lnorg. Chem., 2022, 61, 414–421 CrossRef CAS PubMed.
- R. Liu, K. T. Tan, Y. Gong, Y. Chen, Z. Li, S. Xie, T. He, Z. Lu, H. Yang and D. Jiang, Chem. Soc. Rev., 2021, 50, 120–242 RSC.
- G. S. Pawley, J. Appl. Crystallogr., 1981, 14, 357–361 CrossRef CAS.
- T. F. Willems, C. H. Rycroft, M. Kazi, J. C. Meza and M. Haranczyk, Microporous Mesoporous Mater., 2012, 149, 134–141 CrossRef CAS.
- X. Wang, X. Han, J. Zhang, X. Wu, Y. Liu and Y. Cui, J. Am. Chem. Soc., 2016, 138, 12332–12335 CrossRef CAS PubMed.
- H. Wang, H. Wang, Z. Wang, L. Tang, G. Zeng, P. Xu, M. Chen, T. Xiong, C. Zhou, X. Li, D. Huang, Y. Zhu, Z. Wang and J. Tang, Chem. Soc. Rev., 2020, 49, 4135–4165 RSC.
- R.-J. Wei, M. Xie, R.-Q. Xia, J. Chen, H.-J. Hu, G.-H. Ning and D. Li, J. Am. Chem. Soc., 2023, 145, 22720–22727 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods, single crystal structure details, structure modelling, porosity, TGA, XPS, SEM, and band gap characterization. CCDC 2307737. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cc05650c |
| This journal is © The Royal Society of Chemistry 2024 |