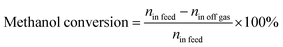Effect of the Si/Al ratio on the performance of hierarchical ZSM-5 zeolites for methanol aromatization
Yan Gaoa,
Binghui Zhengb,
Guang Wu*a,
Fangwei Maa and
Chuntao Liu*a
aSchool of Chemistry and Materials Sciences, Heilongjiang University, Key Laboratory of Chemical Engineering Processes & Technology for High-efficiency Conversion (College of Heilongjiang Province), Harbin 150080, China. E-mail: wu.guang@163.com
bChina Tianchen Engineering Corporation (TCC), Harbin, 150076, China
First published on 29th August 2016
Abstract
A series of hierarchical ZSM-5 zeolites with different Si/Al ratios were successfully prepared using a seed-deduced method and the addition of organosilicone reagents into the medial synthesis system. The crystallinity, morphology, chemical composition, acidity and textural property of the ZSM-5 zeolites were characterized using XRD, SEM, TEM, N2 adsorption, 27Al MAS NMR and NH3-TPD. The catalytic performance was evaluated in terms of methanol aromatization to investigate the effects of the Si/Al ratio on the catalyst stability and coke formation. The results indicated that despite their large external surface area and mesopore volume, a decrease in the Si/Al ratio resulted in an increase in the acidity of the zeolites, which significantly increased the coke formation and decreased their catalytic stability. The acid amount exhibits good linear correlation with the coke content, and the generalized and quantitative correlations are reported.
Introduction
Light aromatic compounds (i.e., benzene, toluene and xylene, which are referred to as simply BTX) are the important fundamental chemicals in the medicinal, perfumery and dyestuff industries. Traditionally, BTX are primarily derived from naphtha reforming and oil thermal cracking. Production of BTX via the traditional route cannot reach the market demand for BTX due to the shortage of fuel oil and the fast growth rate in the BTX demand. Therefore, another efficient route should be developed to increase BTX production.1,2 In recent years, the conversion of methanol to aromatics (MTA) has attracted much attention because MTA is considered an effective route for using methanol and increasing BTX production. Therefore, a high-efficiency catalyst must be developed for the industrial application of MTA.Among the acids used for this reaction, ZSM-5 zeolites were the initial catalysts for the MTA reaction3 and have attracted significant attention due to their good resistance to deactivation by coke and model behaviour in a MTA mechanism study.4–7 However, one of the main drawbacks of the MTA process is related to the deactivation of the catalyst due to the formation of coke, resulting from consecutive reactions between light olefins and Brønsted or Lewis acid sites. This behaviour is due to the limited diffusion rate of the reactants and products within the zeolite materials,8,9 which is a major drawback in most zeolite-catalysed processes. Among several efforts to overcome this disadvantage (e.g., syntheses of nanosized zeolites,10 ultralarge pore zeolites and ordered mesoporous materials),11–13 the synthesis of hierarchical zeolites with a broad pore size distribution is considered the best approach and has attracted much attention.14,15 In the current study, a novel strategy for the synthesis of hierarchical nanocrystalline ZSM-5 was developed based using organosilicone reagents (OSA) to assist the generation of mesopores along with the seed-induced crystallization method.
Despite the fact that some significant advances have been achieved, the catalytic reactivity and/or stability of the catalyst for the MTA reaction must be further enhanced. The formation of aromatics from methanol is a complicated process that involves several reactions catalysed by Lewis and/or Brønsted acids, such as the dehydration of methanol, formation of a hydrocarbon pool and production of aromatics and by-products.16–19 No consensus regarding the relationship between the catalyst properties and the catalytic reactivity has been achieved. Therefore, the relationship between the catalyst properties, especially the acidic properties, and its performance must be understood in detail to develop an efficient catalyst. For example, Choudhary et al.20 and Song et al.21 suggested that the strong acid sites of the HZSM-5 zeolite acted as active sites for the production of aromatics and coke in the conversion of light hydrocarbons. Therefore, the reduction of strong acid sites favoured the suppression of coke but also led to the loss of aromatization activity. Song et al.21 also demonstrated that coke formed on both weak and strong acid sites in butylene aromatization. In addition, the stability of the catalyst was closely related to the acidic distribution. Aguayo et al.22 reported that the high acidic strength of the zeolite catalyst could lower the energy barrier and increase the reaction rate. It is important to note that external surface acid sites and the connectivity of mesopores in the hierarchical zeolites can also significantly contribute to the catalytic performance of zeolite catalysts.
In this study, hierarchical ZSM-5 zeolites with Si/Al ratios ranging from 30 to 80 were prepared using the seed-deduced method and the addition of organosilicone reagents. The catalytic life of the hierarchical ZSM-5 zeolites was evaluated in terms of methanol aromatization. We primarily focused on the effects of the Si/Al ratio on the structure, morphology, acidity, catalytic life and coke formation of the ZSM-5 zeolites. The correlation between the acid content and the coke content are also discussed based on the results.
Experimental
Catalyst preparation
Hierarchical ZSM-5 nanocrystals with different Si/Al ratios were prepared. The seed suspension was prepared as follows: the initial molar composition of the synthesis gel mixtures was x(Al2O3)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100(SiO2)
100(SiO2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17.9(TPAOH)
17.9(TPAOH)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 542(H2O) (x = 1.67, 1.0, 0.77 and 0.625). The calculated amounts of aluminium isopropoxide (AIP) and tetrapropylammonium hydroxide (TPAOH, 25% in water) were mixed with double-distilled water in a polypropylene bottle under vigorous stirring until the AIP was completely dissolved in the solution. Tetraethylorthosilicate (TEOS) was added dropwise to the resulting mixture. The obtained mixture was continuously stirred at room temperature for 3 h. Then, the gel was crystallized at 140 °C for 9 h in a stainless steel autoclave with a Teflon lining to yield the seed suspension.
542(H2O) (x = 1.67, 1.0, 0.77 and 0.625). The calculated amounts of aluminium isopropoxide (AIP) and tetrapropylammonium hydroxide (TPAOH, 25% in water) were mixed with double-distilled water in a polypropylene bottle under vigorous stirring until the AIP was completely dissolved in the solution. Tetraethylorthosilicate (TEOS) was added dropwise to the resulting mixture. The obtained mixture was continuously stirred at room temperature for 3 h. Then, the gel was crystallized at 140 °C for 9 h in a stainless steel autoclave with a Teflon lining to yield the seed suspension.
Sodium aluminates (NaAlO2), distilled water, and NaOH were homogeneously mixed, and then, a silica gel (30% SiO2 in water) solution was dropped into the NaAlO2 and NaOH mixture with vigorous stirring. The Si/Al ratios of the precursor gels were the same as those of the seed suspensions. The unpurified 5.0 wt% seed suspension was added to the reaction mixture and stirred for 10 min. Then, phenylaminopropyltrimethoxysilane (SiO2 weight percentage of 5%) was slowly added to the mixture with agitation at 90 °C for 6 h. Finally, these mixtures were hydrothermally treated at 180 °C for 24 h. The final products were filtered, washed repeatedly with deionized water, dried overnight at 100 °C and calcined for 6 h at 550 °C. The calcined samples were converted to the NH4+ form by ion exchange with a 1 M NH4NO3 solution. The samples in the NH4+ form were calcined again in flowing air at 500 °C for 3 h to afford the H-form products. The synthesized ZSM-5 nanocrystals with different Si/Al ratios are referred to as HZSM5-x, where x is 30, 50, 65, and 80.
Characterization
The powder X-ray diffraction (XRD) patterns of the synthesized zeolite samples were recorded on an ARL X'TRA powder diffractometer using Cu-K radiation (=1.54 Å). The morphology of the samples was examined using scanning electron microscopy (SEM) on a Leo 1430 microscope. The transmission electron microscopy (TEM) images were recorded on a Tecnai G220 S-Twin transmission electron microscope that operated at an acceleration voltage of 200 kV. The physisorption of N2 at 77 K was carried out on a Quantachrome AUTOSORB-1-MP adsorption analyser. Prior to adsorption, the samples were outgassed at 300 °C for 3 h. The chemical compositions of the samples were determined by X-ray fluorescence (XRF) spectroscopy on a SRS-3400 X-ray fluorometer. A Bruker Avance-300 nuclear magnetic resonance (NMR) spectrometer operating at either 104.2 or 122 MHz with a 4 mm MAS probe was used to collect the 27Al NMR spectra. The NH3-TPD experiments were carried out with a conventional setup equipped with a thermal conductivity detector. The TPD profiles were obtained over a temperature range of 120 to 600 °C at a constant heating rate of 10 °C min−1 with a He flow rate of 40 ml min−1.A partially coked sample was used for thermogravimetric analysis (TG) and N2 adsorption measurements. The TG profiles were recorded on a TG 1700 instrument (Perkin-Elmer Corp.). The coked catalyst sample (0.015 g) was heated from room temperature to 700 °C in an air stream at a heating rate of 10 °C min−1. The flow rate of air was 30 ml min−1. In the recorded profiles, the weight loss prior to 300 °C was due to the desorption of water. The weight loss between 300 and 700 °C was caused by burning-off the coke and taken as the total coke content. N2 adsorption measurements were measured at 77 K using the procedure described above. The micropore volume was determined from the adsorption isotherm at a low pressure according to the t-plot method. The amount of internal coke in the zeolite micropore was determined from the decrease in the micropore volume compared to that of a pristine sample. This analysis is based on the assumption that the remaining micropore volume is fully accessible to the N2 molecules through the three-dimensionally interconnected zeolite channels.23,24 The internal coke content was calculated with the assumption that the coke density was 1.22 g cm−3. This density is based on a C/H ratio of 1.25, which is similar to that of coal.25 The amount of external coke (i.e., coke deposition on the external surface) was calculated by subtracting the internal coke content from the total coke content.
Catalytic tests
1.0 g catalyst particles were packed into a stainless steel tubular fixed-bed reactor with an inner diameter of 1.0 cm. Methanol was pumped into the reactor by a metering pump. The MTA reaction was carried out at 673 K with a WHSV of 10.0 h−1 at atmospheric pressure with a 40 ml min−1 N2 flow until a targeted conversion level of 85% was reached. Subsequently, the reactor was purged with high-purity N2 gas (flow rate of 50 ml min−1) for 2 h at 400 °C to remove organic volatiles. A portion of the deactivated catalyst was removed for coke analysis.The reactor exit stream was separated into gas, liquid hydrocarbon and water fractions using an ice-cooled condenser. The gas fraction was analysed on-line using a GC (Agilent 7890A) fitted with Propake-Q columns and a thermal conductivity detector (TCD). The liquid hydrocarbons were analysed using a GC (Agilent 7890A) fitted with a capillary column (HP-5) and an FID. The atomic carbon balance between the inlet (carbon in methanol) and the exit (the sum of carbon in all identified species including unreacted methanol) streams under a steady state run was typically within 15%, and the difference was due to coke formation and carbon deposition. The methanol conversions were calculated based on the carbon balance using the following formula.
Results and discussion
Synthesis and structural characterization of the hierarchical ZSM-5 zeolites
XRD was employed to explore the phase composition and concentration of the constituent phases, and the XRD patterns of the synthesized zeolites are shown in Fig. 1. The XRD peaks of HZSM-5 (2θ = 7.8°, 8.7°, 23.1°, 23.8° and 24.3°) were detected in all of the catalysts, and no impurity peaks were observed. This result indicates that all four zeolites exhibit MFI topology and high purity. The degree of crystallinity of the hierarchical ZSM-5 zeolites with different Si/Al ratios was determined from the peak area between 2θ = 22–25° based on the standard HZSM5-30 sample, which was used as a reference, and the results are listed in Table 1. Under the current synthetic conditions, HZSM5-50 and HZSM5-65 exhibited higher relative crystallinity than HZSM5-30, and HZSM5-80 exhibited lower relative crystallinity, which suggests that the synthesis of hierarchical ZSM-5 with a high (>80) Si/Al ratio is difficult with the current method.| Samples | Relative crystallinity (%) | Si/Al ratio | Surface area (m2 g−1) | Pore volume (cm3 g−1) | ||||
|---|---|---|---|---|---|---|---|---|
| BETa | Microporeb | External | Totalc | Microporeb | Mesopore | |||
| a BET method.b t-Plot method.c Volume adsorbed at p/p0 = 0.99. | ||||||||
| HZSM5-30 | 100 | 19.5 | 379.6 | 226.0 | 153.6 | 0.26 | 0.10 | 0.15 |
| HZSM5-50 | 105 | 33.9 | 324.6 | 182.6 | 142.0 | 0.24 | 0.08 | 0.16 |
| HZSM5-65 | 124 | 42.6 | 316.1 | 174.2 | 142.0 | 0.22 | 0.08 | 0.12 |
| HZSM5-80 | 86 | 57.8 | 321.2 | 165.8 | 155.4 | 0.20 | 0.08 | 0.12 |
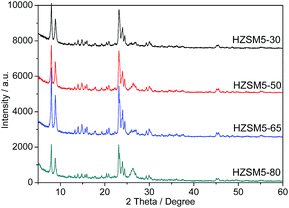
SEM and TEM were used to understand the morphology and crystal size of the zeolite samples. The SEM and TEM images of the HZSM-5 zeolites with different Si/Al ratios are shown in Fig. 2 and 3. The HZSM-5 zeolites have a cubical shape and individual crystal sizes of approximately 50–80 nm. The HZSM-5 zeolites tend to agglomerate into microsized agglomerates due to their high surface Gibbs energy, and these microsized zeolites appear to simply stack loosely together to form larger stacks with visually large spaces between the individual crystals. The influence of the amounts of organosilanes agent (OSA) on the pore characteristics, crystallinity and morphology of the ZSM-5 zeolites was studied according to the reported similar procedures proposed by our research group.26 The formation of zeolite nanocrystals and mesopores is due to the restrained growth of the subnanocrystal zeolite resulting from the addition of OSA. The mesopores were formed primarily from the packing of the nanocrystalline, which is in agreement with the OSA mechanism reported by Xu.27 The TEM images (Fig. 3) confirmed the loose stacking of the crystals. Therefore, HZSM-5 may possess a larger mesopore volume due to particle stacking.
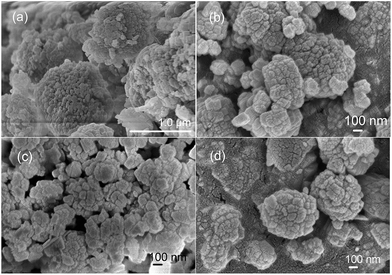 | ||
| Fig. 2 SEM images of hierarchical HZSM-5 zeolites: (a) HZSM5-30, (b) HZSM5-50, (c) HZSM5-65 and (d) HZSM5-80. | ||
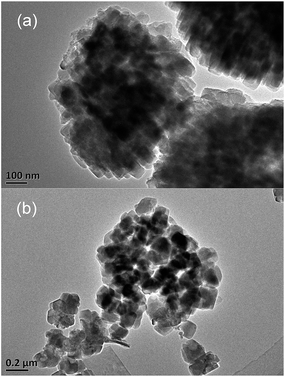
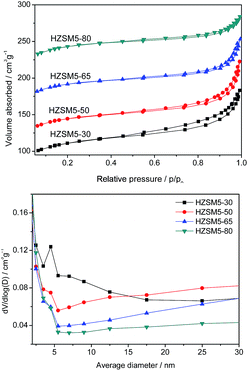
The N2 adsorption/desorption isotherms and BJH pore size distributions for the different catalysts are shown in Fig. 4. The surface areas and micropore volumes of these zeolites, which are listed in Table 2, were calculated using the BET and t-plot methods, respectively. The micropore surface value, which was estimated using the t-plot method, decreased gradually as the Si/Al ratio increased. The isotherms of the hierarchical HZSM-5 samples exhibited a continuous increase in the adsorbed volume with p/p0 and a small hysteresis branch corresponding to adsorption–desorption in the mesopores. These mesopores, which have a ∼4 nm diameter (Fig. 4(b)) for HZSM5-30 sample, correspond to intercrystalline voids. However, the other three samples display wide distribution of mesopores. An increase in the adsorbed volume can be observed from p/p0 = 0.9, which indicates the presence of a small number of large mesopores that are most likely intercrystalline. The values of the micropore volume are quite similar for the four zeolites. However, the samples with low Si/Al ratios exhibited large mesopore volumes.
| Sample | FAl/% (δ = ∼54 ppm) | EFAl/% (δ = ∼0 ppm) |
|---|---|---|
| HZSM5-30 | 93.8 | 6.2 |
| HZSM5-50 | 100 | 0 |
| HZSM5-65 | 100 | 0 |
| HZSM5-80 | 100 | 0 |
Table 1 summarizes the results of the physical and chemical characterization of the samples. The Si/Al ratios of the zeolites are lower than those in the precursor gels and depend on the initial gel composition. This result is due to the consumption of aluminium that provides nucleation sites during the formation of the ZSM-5 structure, which is consistent with the results reported by Kim.28,29 In addition, as the Si/Al ratios of the zeolites increased, the discrepancy between the Si/Al ratio in the gel and in the zeolite increased, which indicates that the additional Al ions in the precursor gels are more effective at increasing the aluminium content in the zeolite framework. This result is in agreement with previous results reported by Migliori.30
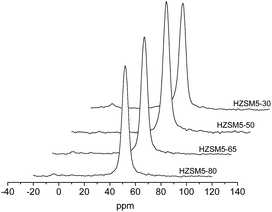
To confirm the environment of the aluminium on a molecular level, 27Al MAS NMR was performed on all of the HZSM-5 samples (shown in Fig. 5). In the 27Al NMR spectra of the HZSM5-50, HZSM5-65 and HZSM5-80 samples, only one signal at 54 ppm was observed, corresponding to tetrahedral Al (FAl) entering the zeolite framework. This result indicates that all of the aluminium is incorporated into the HZSM-5 zeolite framework. In the 27Al NMR spectra of the HZSM5-30 sample, a very small signal with chemical shifts at 0 ppm that correspond to extraframework octahedral Al atoms (EFA) was observed. The EFAl species are assumed to be oligomeric alumina with a low degree of hydration/hydroxylation.31 The FAl contents of the zeolites were estimated from the FAl integrated peak intensities in the 27Al MAS NMR spectra and are listed in Table 2. Approximately 6.2% of EFA was present in the HZSM5-30 sample.
Acidity of the hierarchical ZSM-5 zeolites
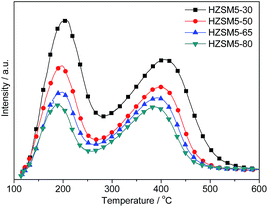
The NH3-TPD profiles of the HZSM-5 samples are shown in Fig. 6. All of the samples exhibited two NH3 desorption peaks. The low-temperature desorption peak (LT peak) and high-temperature desorption peak (HT peak) in the NH3-TPD curves of HZSM-5 correspond to weak acid sites and strong Brønsted acid sites, respectively.32 The density of the Brønsted acid sites, which is the most interesting site for studying the catalytic mechanism, has been assigned to the density of the framework aluminium ions.33 In general, the weak acid sites result from the non-framework Al or the strong adsorption of NH3 to Si–OH in the structural defects.34 As shown in Table 3, slight shifts towards lower temperatures were observed for both the HT and LT peaks in the NH3-TPD profiles as the Si/Al ratios increased. The temperature of the maximum desorption rate on the TPD curve was not regarded as a universally comparable characteristic because it depends on both the nature of the solid sample and the experimental conditions. In addition, the total amounts of desorbed ammonia and the quantitative information for the numbers of strong and weak acid sites are provided in Table 3. As expected, a monotonic decrease in the total acidity (i.e., strong and weak acid sites) was observed as the Si/Al ratios increased. The 27Al NMR spectrum of HZSM-5 revealed that nearly all of the Al atoms were framework Al. Therefore, the weak acid sites correspond to the Si–OH in the structural defects. In combination with the NH3-TPD data, HZSM-5 with higher Si/Al ratios possess fewer Si–OH moieties in the structural defects.| Samples | Tpeak (°C) | Acid amounta (mmol g−1) | |||
|---|---|---|---|---|---|
| LT peakb | HT peakb | Total acidity | Weak acidity | Strong acidity | |
| a The LT peak represents a low temperature desorption peak. The HT peak represents a high temperature desorption peak.b Calculated with Gaussian function fit. | |||||
| HZSM5-30 | 204.0 | 407.5 | 3.28 | 1.33 | 1.95 |
| HZSM5-50 | 194.9 | 398.3 | 1.69 | 0.65 | 1.04 |
| HZSM5-65 | 195.0 | 392.7 | 1.65 | 0.63 | 1.02 |
| HZSM5-80 | 188.0 | 387.0 | 1.34 | 0.50 | 0.84 |
Catalytic performance
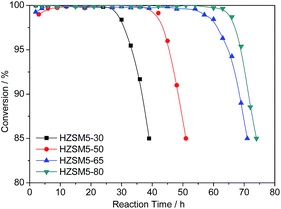
The catalytic performances of the hierarchical HZSM-5 zeolites were tested for the conversion of methanol to aromatics. The methanol conversions of the four catalysts are shown in Fig. 7. At 400 °C, the conversion of the four samples reached 100% after several hours and had been holding it for a period. For the samples with Si/Al ratios of 30, 50, 65 and 80, the period corresponding to a conversion of >99% was 27 h, 42 h, 54 h, and 63 h, respectively. These results indicate that the catalytic lifetime of the hierarchical HZSM-5 zeolite increased as the Si/Al ratio increased. As shown in Table 1, only slight differences in the BET surface areas and pore volumes were observed among the four samples with different Si/Al ratios. Therefore, these values are not major factors that result in different catalytic lifetimes. Based on the morphology of the four samples (Fig. 3 and 4), the particle sizes increased slightly as the Si/Al ratio increased. The large particle size will extend the reactant diffusion path, reducing the catalyst life. Therefore, the difference in the particle sizes is not the primary factor that affects the catalytic activity. The main difference in the four samples is the acidity of the samples. The NH3-TPD analysis results indicate that the number of acid sites decreased significantly as the Si/Al ratio increased. These results indicate that the acidity is the main factor that is responsible for the difference in the catalyst lifetimes. In addition, the appropriate reduction in the number of acid centres is beneficial for improving resistance to carbon deposition.According to the MTA reaction mechanism,35,36 the strong Brønsted acid sites are the active sites for the MTA reaction.37 Many studies have reported that an increase in the acid site strength and concentration can improve the aromatization activity.38,39 However, an increase in the number of acid sites has improved not only the olefin cyclization reaction but also the deep alkylation reaction of the aromatic products, resulting in an increase in the amount of polyalkylaromatics in the reaction system. The polyalkylaromatics may become concentrated and further react with a carbocation to form coke in the micropores if they cannot diffuse quickly out of the molecular sieve channel. Based on this analysis, a simple increase in the number of acid sites does not necessarily improve the catalytic lifetime but simultaneously increases the diffusivity and acidity.
The detailed product selectivities of the MTA reaction at 18 h are provided in Table 4. Methane is produced by the demethylation of aromatic compounds and carbon precursors.40 Therefore, an increase in the carbon deposition leads to more demethylation reactions, resulting in increased methane formation. Some researchers have used changes in the methane content as an indicator to measure catalyst deactivation (i.e., a decrease in the methane content implies a decrease in the carbon deposition rate). The methane selectivity of the hierarchical HZSM-5 zeolites decreased as the Si/Al ratio increased. This result is consistent with the catalytic lifetime result. The selectivities to C2–C5 increased but the selectivities to C7–C9+ decreased as the Si/Al ratio increased. This result suggests that the liquid product yields decreased as the acid density decreased. In addition, the low selectivity to C9+ for the high Si/Al ratio zeolite is the primary reason for the long catalytic lifetime.
| Samples | Product distribution (%) | Aromatic selectivity (%) | BTX selectivity in aromatic (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CH4 | C2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) |
C3![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) |
C2 and C3 | C4 | C5 | C6 | C7 | C8 | C9+ | |||
| HZSM5-30 | 8.1 | 5.4 | 10.0 | 9.3 | 13.6 | 6.3 | 3.4 | 9.0 | 19.2 | 15.7 | 84.6 | 56.3 |
| HZSM5-50 | 7.9 | 5.0 | 12.2 | 9.9 | 12.9 | 6.8 | 6.7 | 12.2 | 17.5 | 8.9 | 74.4 | 65.7 |
| HZSM5-65 | 6.1 | 4.8 | 12.5 | 12.3 | 18.2 | 9.1 | 5.4 | 10.5 | 13.2 | 7.8 | 72.1 | 58.1 |
| HZSM5-80 | 5.1 | 4.1 | 15.2 | 14.7 | 16.9 | 12.8 | 2.5 | 7.9 | 13.9 | 6.9 | 66.9 | 54.6 |
The aromatic selectivities decreased as the Si/Al ratio increased. Methanol aromatization occurs via the initial conversion of methanol into small hydrocarbons, which are then transformed into naphthenes via olefin cyclization. The key process involves olefin cyclization on strong Brønsted acid sites. The low aromatic selectivity of zeolites with high Si/Al ratios is due to the low acid density. The BTX selectivities increased and then decreased as the Si/Al ratio increased. HZSM5-50 exhibited the highest selectivity to BTX compared to that of the other samples. Although the BTX selectivity of HZSM5-30 is higher than those of the other samples, the HZSM5-30 sample has an acid density that is too high, which improves deep alkylation reactions that lead to a decrease in BTX selectivity and increase in C9+ selectivity. The reason for the decrease in the BTX selectivity for HZSM5-65 and HZSM5-80 is the increase in the non-aromatic selectivity (e.g., cyclohexane and isohydrocarbon). These results suggest that the optimal Si/Al ratio is necessary to increase the BTX selectivity.
Analysis of the coke and partially deactivated catalysts
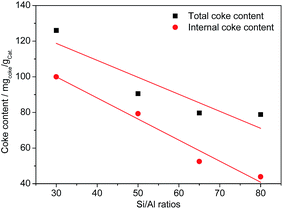
The coke content, lost BET surface area and pore volume of the partially deactivated catalysts (conversion dropped to 85%) were characterized by TG and N2 adsorption/desorption analyses (Table 5). The amounts of BET surface area and pore volume that were lost are significant even though the conversions of methanol still remained high. This result suggests that a small amount of coke can substantially decrease the BET surface areas and pore volumes. The same trends for the total coke contents, internal coke contents and micropore surface areas were observed. The amounts of micropore surface areas and internal coke contents that were lost linearly decreased with increasing Si/Al ratios. The HZSM5-30 and HZSM5-50 samples exhibited a larger loss in the BET surface areas than that of the HZSM5-65 and HZSM5-80 samples even though HZSM5-30 and HZSM5-50 possessed large mesopore volumes and external surface areas. This result is due to the HZSM5-30 and HZSM5-50 samples possessing more acid sites and stronger acidity. Fig. 8 shows the proportional relationship between coke formation and the Si/Al ratios. In addition, up to a Si/Al ratio of 80, the catalytic performance of the HZSM5 catalysts can be linearly correlated with the amount of coke. This result suggests that a decrease in the acid density in the experimental range may result in higher catalyst stability because strong acid sites play a significant role in coke formation.| Samples | Coke contents (mgcoke gcat.−1) | Surface area (m2 g−1) | Pore volume (cm3 g−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Totala | Internalb | External | BET | Micropore | External | Total | Micropore | Mesopore | |
| a Measured by TG.b Calculated by (micropore volume of fresh catalyst − micropore volume of deactivated catalyst) cm3 g−1 × 1.22 g cm−3 × 1000. | |||||||||
| HZSM5-30 | 126.0 | 100.0 | 26.0 | 293.1 | 181.4 | 111.7 | 0.15 | 0.08 | 0.07 |
| HZSM5-50 | 90.5 | 79.3 | 11.2 | 250.9 | 146.1 | 104.8 | 0.13 | 0.07 | 0.06 |
| HZSM5-65 | 79.7 | 52.5 | 27.2 | 159.2 | 83.9 | 75.4 | 0.08 | 0.04 | 0.04 |
| HZSM5-80 | 78.8 | 43.9 | 34.9 | 163.7 | 56.7 | 107.0 | 0.10 | 0.04 | 0.06 |
Conclusions
Hierarchical ZSM-5 zeolites with Si/Al ratios ranging from 30 to 80 were successfully synthesized by a seed-deduced and organosilicone reagent-assisted method. The Si/Al ratio of the hierarchical ZSM-5 zeolite exerted an important influence on the structure, morphology and acidity of the ZSM-5 zeolites as well as the catalytic lifetime and coke formation in the MTA reaction. The high acid density and acid strength resulted in a higher selectivity to aromatic compounds. The hierarchical ZSM-5 zeolite with a Si/Al ratio of 50 exhibited the highest selectivity to BTX due to the catalyst possessing the appropriate acid density and acid strength. The sample with a low Si/Al ratio exhibited a long catalytic lifetime due to the low acid density and good resistance to carbon deposition. A linear relationship was observed between the Si/Al ratio and the coke content.Acknowledgements
This work was financially supported by the Postdoctoral Fund of the Heilongjiang Province of China (No. LBH-Z14208) and the open foundation of the Key Laboratory of Functional Inorganic Material Chemistry (Heilongjiang University), Ministry of Education, China.Notes and references
- M. Zhang, S. Xu, J. Li, Y. Wei, Y. Gong, Y. Chu, A. Zheng, J. Wang, W. Zhang, X. Wu, F. Deng and Z. Liu, J. Catal., 2016, 335, 47 CrossRef CAS.
- R. Khare and A. Bhan, J. Catal., 2015, 329, 218 CrossRef CAS.
- T. V. W. Janssens, J. Catal., 2009, 264, 130 CrossRef CAS.
- H. Yamazaki, H. Shima, H. Imai, T. Yokoi, T. Tatsumi and J. N. Kondo, Angew. Chem., Int. Ed., 2011, 50, 1853 CrossRef CAS PubMed.
- M. Bjøgen, S. Svelle, F. Joensen, J. Nerlov, S. Kolboe, F. Bonino, L. Palumbo, S. Bordiga and U. Olsbye, J. Catal., 2007, 249, 195 CrossRef.
- L. Palumbo, F. Bonino, P. Beato, M. Bjøgen, A. Zecchina and S. Bordiga, J. Phys. Chem. C, 2008, 112, 9710 CAS.
- J. Kim, M. Choi and R. Ryoo, J. Catal., 2010, 269, 219 CrossRef CAS.
- F. J. Keil, Microporous Mesoporous Mater., 1999, 29, 49 CrossRef CAS.
- M. Stöcker, Microporous Mesoporous Mater., 1999, 29, 3 CrossRef.
- J. Li, P. Miao, Z. Li, T. He, D. Han, J. Wu, Z. Wang and J. Wu, Energy Convers. Manage., 2015, 93, 259 CrossRef CAS.
- A. Corma, M. Díaz-Cabańas, J. Martinez-Triguero, F. Rey and J. Rius, Nature, 2002, 418, 514 CrossRef CAS PubMed.
- J. L. Paillaud, B. Harbuzaru, J. Patarin and N. Bats, Science, 2004, 304, 990 CrossRef CAS PubMed.
- H. Gies, Nature, 2005, 437, 633 CrossRef CAS PubMed.
- J. Pérez-Ramírez, C. H. Christensen, K. Egeblad, C. H. Christensen and J. C. Groen, Chem. Soc. Rev., 2008, 37, 2530 RSC.
- K. Egeblad, C. H. Christensen, M. Kustova and C. H. Christensen, Chem. Mater., 2008, 20, 946 CrossRef CAS.
- S. Ilias and A. Bhan, ACS Catal., 2012, 3, 18 CrossRef.
- U. Olsbye, S. Svelle, M. Bjorgen, P. Beato, T. V. W. Janssens, F. Joensen, S. Bordiga and K. P. Lillerud, Angew. Chem., Int. Ed., 2012, 51, 5810 CrossRef CAS PubMed.
- Y. Q. Song, X. X. Zhu and L. Y. Xu, Catal. Commun., 2006, 7, 218 CrossRef CAS.
- T. Wang, X. P. Tang, X. F. Huang, W. Z. Qian, Y. Cui, X. Y. Hui, W. Yang and F. Wei, Catal. Today, 2014, 233, 8 CrossRef CAS.
- T. V. Choudhary, A. Kinage, S. Banerjee and V. R. Choudhary, Catal. Commun., 2006, 7, 166 CrossRef CAS.
- Y. Song, X. Zhu, S. Xie, Q. Wang and L. Xu, Catal. Lett., 2004, 97, 31 CrossRef CAS.
- A. T. Aguayo, A. G. Gayubo, R. Vivamco, M. Olazar and J. Bilbao, Appl. Catal., A, 2005, 283, 197 CrossRef CAS.
- S. S. Kim, J. Shah and T. J. Pinnavaia, Chem. Mater., 2003, 15, 1664 CrossRef CAS.
- L. Zhang, C. Yang, X. Meng, B. Xie, L. Wang, L. Ren, S. Ma and F. S. Xiao, Chem. Mater., 2010, 22, 3099 CrossRef CAS.
- I. Schmidt, C. Madsen and C. J. H. Jacobsen, Inorg. Chem., 2000, 39, 2279 CrossRef CAS PubMed.
- Y. Gao, G. Wu, F. Ma, C. Liu, F. Jiang, Y. Wang and A. Wang, Microporous Mesoporous Mater., 2016, 226, 251 CrossRef CAS.
- C. Xu, H. Liu, M. Jia, J. Guan, S. Wu, T. Wu and Q. Kan, Appl. Surf. Sci., 2011, 257, 2448 CrossRef CAS.
- S. D. Kim, S. H. Noh, J. W. Park and W. J. Kim, Microporous Mesoporous Mater., 2006, 92, 181 CrossRef CAS.
- S. D. Kim, S. H. Noh, K. H. Seong and W. J. Kim, Microporous Mesoporous Mater., 2004, 72, 185 CrossRef CAS.
- M. Migliori, A. Aloise and G. Giordano, Catal. Today, 2014, 227, 138 CrossRef CAS.
- D. Ma, Y. Lu, L. Su, Z. Xu, Z. Tian, Y. Xu and L. Lin, J. Phys. Chem. B, 2002, 106, 8524 CrossRef CAS.
- N. Katada, H. Igi, J.-H. Kim and M. Niwa, J. Phys. Chem. B, 1997, 101, 5969 CrossRef CAS.
- D. J. Parrillo, C. Lee and R. J. Gorte, Appl. Catal., A, 1994, 110, 67 CrossRef CAS.
- S. Kouva, J. Kanervo, F. Schubler, R. Olindo, J. A. Lercher and O. Krause, Chem. Eng. Sci., 2013, 89, 40 CrossRef CAS.
- S. Ilias, R. Khare, A. Malek and A. Bhan, J. Catal., 2013, 303, 135 CrossRef CAS.
- F. L. Bleken, K. Barbera, F. Bonino, U. Olsbye, K. P. Lillerud, S. Bordiga, P. Beato, T. V. W. Janssens and S. Svelle, J. Catal., 2013, 307, 62 CrossRef CAS.
- T. V. Choudhary, A. Kinage, S. Banerjee and V. R. Choudhary, Catal. Commun., 2006, 7, 166 CrossRef CAS.
- J. F. Haw, W. Song, D. M. Marcus and J. B. Nicholas, Acc. Chem. Res., 2003, 36, 317 CrossRef CAS PubMed.
- Y. Yang, C. Sun, J. Du, Y. Yue, W. Hua, C. Zhang, W. Shen and H. Xu, Catal. Commun., 2012, 24, 44 CrossRef CAS.
- J. F. Haw, W. Song, D. M. Marcus and J. B. Nicholas, Acc. Chem. Res., 2003, 36, 317 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |