Interactions of flavan-3-ols and procyanidins with membranes: mechanisms and the physiological relevance
Sandra V.
Verstraeten
a,
Cesar G.
Fraga
b and
Patricia I.
Oteiza
*cd
aDepartment of Biological Chemistry and IQUIFIB (UBA-CONICET), Buenos Aires, Argentina
bPhysical Chemistry and IBIMOL (UBA-CONICET), School of Pharmacy and Biochemistry, University of Buenos Aires, Buenos Aires, Argentina
cDepartment of Nutrition, University of California, Davis, One Shields Avenue, Davis, CA 95616, USA
dDepartment of Environmental Toxicology, University of California, Davis, One Shields Avenue, Davis, CA 95616, USA. E-mail: poteiza@ucdavis.edu; Fax: +530-752-8966; Tel: +530-754-6074
First published on 23rd October 2014
Abstract
Flavonoids are a type of phenolic compound widely present in edible plants. A great number of health benefits have been ascribed to flavonoid consumption in the human population. However, the molecular mechanisms involved in such effects remain to be identified. The flavan-3-ols (−)-epicatechin and (+)-catechin, and their related oligomers (procyanidins) have been thoroughly studied because of their capacity to interact with cell membranes. Starting with these interactions, procyanidins could modulate multiple biochemical processes, such as enzyme activities, receptor-ligand binding, membrane-initiated cell signaling, and molecule transport across membranes. This review focuses on molecular aspects of procyanidin interactions with membrane lipid components, and the resulting protection of the membranes against mechanical and/or oxidative damage, resulting in the maintenance of cell functions.
Introduction
Flavonoids are (poly)phenolic compounds synthesized by plants as secondary metabolites. The regular consumption of flavonoid-containing foods and beverages can provide health benefits. In spite of the large amount of research performed up to date, the mechanisms underlying those beneficial effects are not fully understood.The interaction of flavonoids with membrane components, i.e. lipids and proteins, may in part account for flavonoid actions on human and animal health. A direct interaction of flavonoids with membrane proteins could affect membrane-associated processes, such as the regulation of enzymes, channels and receptors, and membrane-initiated cell signaling.1 Flavonoids can also bind to lipids and modulate the biophysical properties of membranes, which ultimately could affect the aforementioned membrane-associated processes.
This review will focus on a particular class of flavonoids, the flavan-3-ols and their oligomers, the procyanidins, which are found in large amounts in fruits (e.g. berries, apples), cocoa, nuts and beans, and derived foods.2 The flavan-3-ol and procyanidin daily intake can significantly vary in different populations (e.g. 455 and 135 mg per day for men in Spain and Greece, respectively).3 Such variations are explained by, among other factors, nutritional habits, the local availability of procyanidin-containing foods, variations within varieties, and seasonal factors. In the United States of America the main dietary sources of procyanidins are apples, cocoa, peanuts, and grapes.4 Cocoa (Theobroma cacao) beans, and peanut (Arachis hypogea L.) and grape (Vitis vinifera) skins contain almost exclusively (−)-epicatechin-derived procyanidins.5–7 The relative content of monomers and procyanidins in cocoa beans was estimated to be 36% of monomers, 17% of dimers and 48% of higher procyanidins (trimers to decamers).8 By comparison, the peanut skin contains 2.5% monomers, 18% dimers, 34% trimers, and 46% tetramers.9 As it will be discussed in the next section, even when cocoa beans and peanut skin procyanidins are built by (−)-epicatechin units, the chemical and three-dimensional structures of these molecules are different in the two plants. These differences may determine distinct interactions with membrane components, and hence different biological effects.
Chemical structure of flavan-3-ols and procyanidins
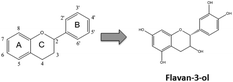
Flavonoids are plant secondary metabolites presenting a huge number of chemical structures. Overall, these structures are characterized by the presence of two aromatic rings (A and B) connected by a three-carbon chain forming an oxygenated heterocycle (C ring) (Fig. 1). Based on the substitutions incorporated on this basic skeleton, such as keto, hydroxyl, methoxyl, or gallate groups, flavonoids are categorized into six families.10 One of them, the flavan-3-ols, results from the incorporation of hydroxyl groups in positions 3, 5, 7, 3′ and 4′ (Fig. 1). Regarding the hydroxyl group in position 3, two spatial conformations are possible. The 2,3-cis isomer is denominated (−)-epicatechin, whereas the 2,3-trans isomer is denominated (+)-catechin (Fig. 2). Both monomers can oligomerize through 4β → 8 bonds (e.g. in cocoa beans) or 2β → O7 bonds (e.g. in peanut skin7,9) to generate the procyanidins, composed of two or more units.11 Procyanidins built up from (−)-epicatechin are more frequently found in nature than those composed of (+)-catechin.12 The biochemical pathway of monomer oligomerization in plants is still not completely elucidated.13 However, it has been proposed that this process requires the oxidation of flavan-3-ols to their respective quinones, which subsequently link to each other forming the procyanidins.12,14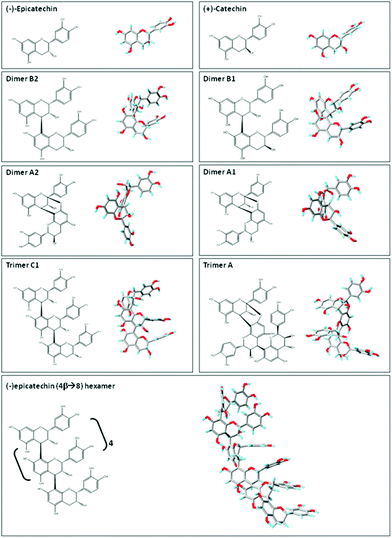 | ||
| Fig. 2 Planar chemical structure and three-dimensional structures of flavan-3-ol and related procyanidins. Planar chemical and three-dimensional structures of the flavan-3-ol and procyanidins: (−)-epicatechin; (+)-catechin; dimer B2 [(−)-epicatechin-(4β → 8) (−)-epicatechin]; dimer B1 [(−)-epicatechin-(4β → 8) (+)-catechin]; dimer A2 [(−)-epicatechin (4β → 8, 2β → O-7) (−)-epicatechin]; dimer A1 [(−)-epicatechin (4β → 8, 2β → O-7) (+)-catechin]; trimer C1 [(−)-epicatechin-(4β → 8) (−)-epicatechin-(4β → 8) (−)-epicatechin]); trimer A [(−)-epicatechin (4β → 8, 2β → O-7) (−)-epicatechin (4β → 8) (−)-epicatechin]; and hexamer procyanidin [(−)-epicatechin-(4β → 8) [(−)-epicatechin-(4β → 8)]4 (−)-epicatechin]. Three-dimensional structures were modeled using PRODRG.83 | ||
Flavan-3-ols and procyanidins are usually represented as planar structures. However, the spatial conformation adopted by these molecules is not planar. In fact, the (−)-epicatechin B ring is oriented almost perpendicularly to the rest of the molecule (Fig. 2). Dimer B2 [(−)-epicatechin (4β → 8) (−)-epicatechin] and dimer B1 [(−)-epicatechin (4β → 8) (+)-catechin] adopt similar U-shaped conformations in which the two B rings from the monomeric units are almost stacked (Fig. 2). By molecular modeling, we observed that the incorporation of an additional 2β → O-7 bond (dimer A2, Fig. 2) between the two (−)-epicatechins generates a double-bonded rigid molecule that does not allow the stacking.15 Worthy of note, the conformation adopted by the dimer A2 [(−)-epicatechin (4β → 8, 2β → O-7) (−)-epicatechin] is more extended than that of the dimer A1 [(−) epicatechin (4β → 8, 2β → O-7) (+)-catechin)] (Fig. 2).15 This observation implies that not only the additional bonds between the monomers, but also the stereochemistry of the hydroxyl group in position 3 is important in determining the overall shape of the molecule. Larger procyanidins, e.g. cocoa hexamer (Fig. 2), adopt extended conformations with (−)-epicatechin monomers arranged in a left-handed helix.14 As mentioned before, differences in the procyanidin spatial conformation will lead to differential interactions of these procyanidins with other molecules, such as lipids, proteins, carbohydrates and nucleic acids.15,16
Depending on the number and characteristics of their substitutions, flavonoids can partition differentially in hydrophilic or lipophilic domains. Among the flavan-3-ols, (+)-catechin and (−)-epicatechin partition almost equally between n-octanol and a buffered water solution.17 The incorporation of a hydroxyl group in the 5′ of (−)-epicatechin defining the (−)-epigallocatechin increases the hydrophilicity of the molecule.17 The solubility of procyanidins in water (buffered solutions) increases with the number of monomer units.17 By molecular dynamics modeling of procyanidin hydration, it was evidenced that the monomers, but not the procyanidins, have a tendency to self-associate following hydrophobic interactions.18 In this regard, Pianet et al.18 postulated that “the higher the degree of polymerization, the greater the number of structural degrees of freedom that favor hydration and prevent intermolecular hydrophobic stacking”, thus explaining the greater solubility of higher procyanidins in water.
Flavan-3-ol and procyanidin interactions with model membranes
A major interest of our research over the last 10 years has been oriented to characterize the interactions of flavan-3-ols and procyanidins with membranes, and to assess the biological/functional relevance of such interactions. Studies were performed using (+)-catechin, (−)-epicatechin and procyanidins purified from cocoa19 and from peanut skins.7The initial experiments were designed to investigate whether the interactions of (−)-epicatechin, (+)-catechin and procyanidins (dimer to hexamer) with membranes could prevent lipid oxidation, given that these compounds have well-recognized antioxidant properties in vitro. Given that the antioxidant ability of flavan-3-ols resides mainly in the presence of the catechol moiety in the B ring,20 we expected that the higher the number of monomer units, the higher would be the antioxidant capacity. Thus, the concentrations of procyanidins were adjusted to achieve equivalent amounts of monomers in the samples. In liposomes composed of phosphatidylcholine (PC) and phosphatidylserine (PS) treated with the generator of lipid-soluble radicals (2,2′-azobis (2,4-dimethylvaleronitrile), AMVN) the antioxidant potential of procyanidins increased linearly with the number of monomer units in the molecule.21 This size-dependent relationship was not observed when using the generator of water-soluble radicals (2,2′-azobis (2-amidinopropane) hydrochloride, (AAPH).22 Both AMVN and AAPH are azo-compounds that upon thermal decomposition generate carbon-centered radicals23 although the site where those radicals are formed is different. Due to its hydrophobicity, AMVN incorporates into lipid bilayers thus generating the radicals within a hydrophobic environment. In contrast, AAPH is hydrophilic and generates oxidizing radicals in the water milieu.23 The different procyanidin behavior against these two oxidants suggested that procyanidins may interact with the membrane surface limiting the access of lipid soluble oxidants to the hydrophobic core of the membrane making larger molecules more efficient when preventing membrane oxidation.21
Interaction of procyanidins with phospholipids
To substantiate the above-mentioned hypothesis, we next investigated whether procyanidins affected membrane physical properties that may limit the accessibility of the oxidants to the core of the lipid bilayer. We observed that procyanidins decreased the surface potential of PC and PS liposomes in a concentration- and chain length-dependent manner.21 The effects of procyanidins on the membrane surface potential depended on the characteristics of the polar headgroup of the phospholipids investigated. Thus, dimer B2 increased the liposome surface potential in liposomes composed of only PC16 and decreased it in liposomes composed of PC and PS.21 This difference could be ascribed to both the overall surface charge and the interactions established between the polar headgroups of phospholipids. While at neutral pH PC is a zwitterion, PS is negatively charged. In addition, neighbor PCs establish only weak interactions between their polar headgroups, whereas the coexistence of PC and PS in the membrane not only generates stronger hydrogen bonds between the polar headgroups but also increases lipid packing.24 Thus, in membranes containing PC and PS the penetration of dimer B2 into the bilayer may be restricted and confined to the surface. In contrast, the more relaxed surface of PC liposomes may allow the dimer B2 to penetrate deeper into the bilayer, exposing the phosphate groups of PC to the water milieu. Supporting this, dimer A1, which resembles dimer B2 in its spatial conformation, also increased the surface potential of PC liposomes.16 A different behavior was observed for dimer A2, a molecule that adopts a conformation more extended than those of dimers B2 and A1, displaying a biphasic effect in PC liposomes.16 Both trimer C1 [(−)-epicatechin (4β → 8) (−)-epicatechin (4β → 8) (−)-epicatechin] and trimer A [(−)-epicatechin (4β → 8, 2β → O-7) (−)-epicatechin (4β → 8) (−)-epicatechin], which adopt extended conformations (Fig. 2), similarly increased the PC liposome surface potential, supporting the hypothesis that their interaction with the membrane occurs at the surface level.Flavan-3-ols and procyanidins can alternatively modulate the membrane fluidity. In liposomes composed of brain PC and containing a fluorescent probe located close to the membrane surface,25 we found that (+)-catechin and (−)-epicatechin have minor effects on the membrane fluidity.17 The effects of (+)-catechin and (−)-epicatechin on the membrane fluidity were also dependent on the chemical characteristics of the phospholipids. In liposomes composed of dipalmitoyl phosphatidylcholine (DPPC, a synthetic phospholipid with two saturated acyl chains), (+) and (−)-catechin as well as (+) and (−)-epicatechin promoted membrane rigidification, although the magnitude of that effect was different for each flavan-3-ol.26 (−)-Epicatechin was the most effective in promoting membrane rigidification not only at the membrane lipid–water interface but also at the hydrophobic core.26 On this basis, it was proposed that these geometrical stereoisomers have different lipophilicity, (−)-epicatechin being the most lipophilic of the series, and thus able to reach the deeper sections of the bilayer.26 In line with this, the hydrophilic flavonoid EGCG mainly interacts with the membrane surface and promotes its rigidification, with almost no effect on the hydrophobic core of the bilayer.27 By molecular dynamics simulations, Sirk et al.28 showed that (−)-epicatechin could adsorb onto 1-palmitoyl-2-oleoyl phosphatidylcholine (POPC) bilayers to a greater extent than EGCG. However, once adsorbed, both flavonoids induced a lateral expansion of the bilayer below the phosphate group of the phospholipid, compressing their immediately adjacent lipids.28 This lateral compression of the membrane surface may account for the rigidifying effect of (−)-epicatechin and EGCG both in dioleoyl phosphatidylcholine (DOPC) and DPPC liposomes (two synthetic phospholipids). The extrapolation of these results to biological membranes should consider that the latter contains a complex mixture of phospholipid species, with different acyl chain lengths and saturation degrees. Consequently, the individual molecules of natural phospholipids will pack differently from that in membranes composed of a single PC species. Thus, measurements would represent the average effect of flavonoids on the different phospholipid species, and underscore the need for cautiousness when extrapolating the results obtained from artificial models to physiological conditions.
In contrast to flavan-3-ol monomers, which have minor effects on the liposome fluidity, dimer B2 and hexamers markedly decreased the fluidity of the membranes.17 Supporting the notion that procyanidin adsorption would underlie their effects on the membrane fluidity, the effect of the procyanidins on the membrane surface potential correlated with their ability to rigidify the membrane.17 Dimers A1 and A2, and trimers A and C1 also decreased the fluidity of the bilayer, mainly by establishing contact with the surface of the membrane.16 It is to be noted that even when the interactions of procyanidins with membranes occurred at the surface, their rigidifying effect propagated into the bilayer and affected the acyl chains of phospholipids.17 Interestingly, the magnitude of the rigidifying effect of the dimers in this region followed the order B2 < A1 ≪ A2 which seems to be associated with the type of folding of their chemical structures. In addition, positive and significant correlations related the effects of the dimers on the membrane fluidity at the surface and the deepest region of the bilayer. By analyzing the slope of those correlations, it can be suggested that the folded dimers affect mainly the surface of the membrane and, to a lesser extent, the hydrophobic core of the membrane.16 In the case of dimer A2 that is more extended and thus potentially able to interact with more phospholipids than the other dimers, the rigidifying effect propagated to a similar extent from the surface down to the hydrophobic core of the bilayer.16 The comparison of the effects of trimers A and C1 on the membrane fluidity shows no significant differences between them, neither at the most superficial nor in the deeper region of the bilayer, thus suggesting that the additional bonding in trimer A has no major impact on the overall interaction of this procyanidin with membrane lipids.16 Supporting this, Yu et al. demonstrated by solid state 2H- and31P-NMR that trimer C2 [(+)-catechin (4β → 8) (+)-catechin (4β → 8) (+)-catechin] which also adopts an extended conformation in solution29 caused minimal alterations in the membrane fluidity, mostly at the surface level.30 Therefore, it is important to consider the spatial conformation of the procyanidins, and not just their chemical structure and/or the number of monomer units in their structure, to understand the consequences of procyanidin interactions with biological membranes.
We next hypothesized that the interaction of procyanidins with membranes might affect the incorporation of hydrophobic compounds into the bilayer. To assess this possibility, we evaluated whether (−)-epicatechin, (+)-catechin, and a series of procyanidins (dimer to hexamer) were capable to prevent the disruption of the bilayer by Triton X-100. This detergent incorporates into the bilayer and causes its disruption in a progressive and controlled manner. The progression of this process can be followed from the changes in the fluorescence of a probe incorporated in the bilayer.31 This method provides a quantitative value (C50) which is inversely related to the susceptibility of the membrane to be disrupted.21 The pre-incubation of liposomes composed of PC and PS with the procyanidins made liposomes more resistant to disruption.21 Experiments were performed using equivalent concentrations of monomers for all the procyanidins assessed. Despite this, the number of monomers in the procyanidins still correlated with the increase in C50 values.21 The comparison of the protective effects of dimers and trimers containing only 4β → 8 bonds versus those having both 4β → 8 and 2β → O-7 bonds indicates that the more extended the three-dimensional structure, the higher the protection against detergent-mediated membrane disruption.16
The above results show that the capacity of procyanidins to maintain the membrane integrity when exposed to a detergent is determined not only by the number of monomers in their structure but also by their three-dimensional configuration.21,22 These protective effects of procyanidins against membrane disorganization positively correlate with their capacity to scavenge free radicals.21 This suggest that the capacity of large procyanidins to limit the accessibility to the membrane of hydrophobic oxidizing compounds defines an indirect antioxidant effect that would make flavan-3-ols and procyanidins physiological protectors of the gastrointestinal tract.10
Interaction of procyanidins with glycolipids
Similar to that discussed before, the capacity of procyanidins to protect membranes from detergent-mediated disorganization relies on the nature of the polar headgroup of membrane lipids. Galactolipids are a family of lipids having a galactose moiety as the polar headgroup (Fig. 3). Galactose could establish hydrogen bonding with the procyanidins, thus maintaining the procyanidins close to the surface of the membrane. The protective capacity of hexameric procyanidins against membrane disruption was markedly higher in galactolipid-containing liposomes than in those containing PC and PS.21 While the presence of a single galactose in galactolipids favors the interaction of procyanidins with the membrane, lipids containing a higher number of sugar moieties impair this interaction. This is the case of complex sphingolipids, e.g. gangliosides, which bear large polar headgroups with four or more sugar moieties. We recently demonstrated such impairment using liposomes containing the asialoganglioside GM1 (aGM1, Fig. 3). This ganglioside bears four carbohydrate moieties in its polar headgroup (two galactose molecules, one glucose and one N-acetyl galactosamine) and is concentrated in certain membrane domains, i.e. the lipid rafts.32 The presence of aGM1 turned liposomes more susceptible to disruption by Triton X-100 or sodium deoxycholate (DCA),33 two amphiphilic but chemically different compounds. Therefore, the protective effects of procyanidins on membranes depend both on the strength and the location of the procyanidin interaction with membrane components rather than on the chemical structure of the stressor. The bulky headgroup of aGM1 protrudes several Å from the membrane surface,34 which might keep procyanidins away from the membrane surface. Conversely, the small headgroup of galactolipids may put procyanidins in close contact with the surface of the membrane allowing them to exert their protective effects. Thus, the relative amount of the different kinds of lipids in biological membranes will determine not only the strength of procyanidin contacts with the membrane but also their location, and ultimately their potential biological actions (Fig. 4).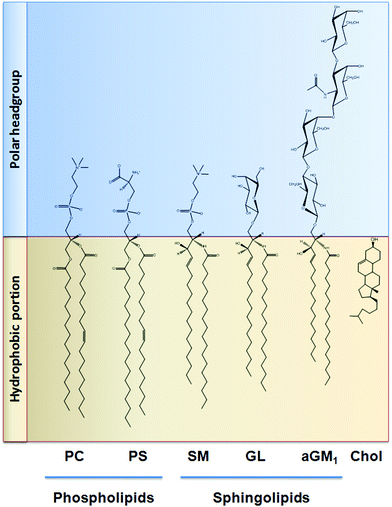 | ||
| Fig. 3 Chemical structure of the lipids discussed in this work. PC: phosphatidylcholine; PS: phosphatidylserine; SM: sphingomyelin; GL: galactolipid; aGM1: asialo ganglioside GM1; Chol: cholesterol. | ||
Interaction of procyanidins with cholesterol
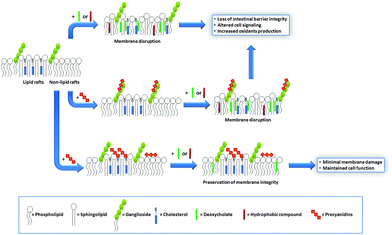
Cholesterol is a key component of the animal cell plasma membrane, and among several other functions it plays a role in the arrangement of membrane lipids.35,36 Cholesterol is particularly abundant in specialized lipid domains of the plasma membrane, the so-called lipid-rafts,37 where it interacts preferentially with sphingomyelin (SM, Fig. 3) rather than with the phospholipids.38 The cholesterol-SM complex adopts a cylindrical, bilayer-forming shape that facilitates lipid raft organization.37 In the absence of cholesterol, hexamers bound to liposomes composed of PC and SM exert a mild protective effect against membrane disruption by sodium DCA or Triton X-100.33 The magnitude of this effect was similar to that previously found in PC liposomes.17 This was not surprising given that, in spite of their different hydrophobic domains, PC and SM share a common polar headgroup, a phosphorylcholine moiety (Fig. 3). For that reason, the nature and magnitude of the interactions between the hexamer and the membrane were similar for both types of liposomes. The presence of cholesterol in liposomes was central to procyanidin–membrane interactions. As observed in liposomes containing equimolar amounts of PC, SM, and cholesterol,39 the capacity of the hexamer to prevent bilayer disruption by detergents was markedly higher with respect to membranes lacking cholesterol.33 Again, the overall protective effect of the hexamer was independent of the detergent used. As we will discuss in the next section, the interaction of the procyanidins with lipid raft components is highly relevant to its biological actions given that lipid rafts concentrate multiple molecules involved in cell signaling.1Altogether, the experimental data obtained from artificial membranes provide mechanistic insights regarding the strength and location of procyanidin binding to membranes, which clearly depends on the characteristics of lipid polar headgroups. Some lipids will favor closer interactions between procyanidins and membranes, shielding them from the aggression by external agents. Others will keep procyanidins far from the surface, decreasing their capacity to protect membranes. Thus, the potential beneficial effects of procyanidins in cells, which have a complex mixture of lipids arranged in dynamic domains, might be higher in those membrane regions containing lipids that favor procyanidins’ contact with the membrane surface, and minimal in those containing lipids that impede procyanidins’ contact with the membrane (Fig. 4).
Interactions of flavan-3-ols and procyanidins with cell membranes
A major logic limitation to the effects of procyanidins on biological membranes is their presence at the appropriate place and in the appropriate amounts. The concentration of procyanidins in the post-prandial gastrointestinal tract is relatively high.6,40 Large procyanidins incorporated through the diet are poorly absorbed by the enterocytes, whereas the monomers, rather than the dimers, can reach the bloodstream and other organs.41,42 For this reason, procyanidins with more than three monomeric units remain in the gastrointestinal tract where they can be found as parent compounds, with the capacity to interact with the apical membrane of gastrointestinal epithelial cells.43,44Interactions of procyanidins with the plasma membrane
Based on our data showing that procyanidins interact with lipids and help in maintaining the integrity of synthetic membranes, we extended the investigation to determine whether a similar protective effect may take place in biological membranes. We used a model of intestinal epithelial cells, the human colorectal adenocarcinoma (Caco-2) cells.45 These cells have the particularity to spontaneously differentiate into polarized, confluent monolayers with functional brush borders and tight cellular junctions.46 Differentiated Caco-2 cells constitute a valuable and extensively used model for the study of intestinal nutrient and drug transport and metabolism.45,47 In differentiated Caco-2 cells, procyanidins interact with the plasma membrane and these interactions depend on the procyanidin degree of polymerization.48 Hexameric procyanidin adsorption occurs within 30 min of cell exposure and the interaction is stable. The latter is supported by findings that after removal of the hexamer from the incubation medium, the protective actions of the procyanidin are still observed.48 Considering this, the average orocecal transit times, and the fact that procyanidins are found along the gastrointestinal tract within 1 and 12 h after consumption,40 it is possible to speculate that a regular ingestion of procyanidin-containing foods could provide sustained health effects.Under physiological conditions, intestinal cells interact with both endogenous and exogenous compounds that potentially might damage their plasma membrane. For example, intestinal cells are exposed to bile salts, a group of endogenous amphiphilic molecules responsible for diet fat emulsification and absorption. One of those compounds is DCA, a secondary bile acid that results from the metabolism of chenodeoxycholic acid by intestinal bacteria. DCA is an amphiphilic molecule that spontaneously intercalates between lipids and potentially damages the integrity of the cell plasma membrane. High chronic concentrations of DCA are associated with an increased risk for colorectal cancer.49
In intestinal cell cultures, DCA caused cell death (necrotic and apoptotic) that was prevented by procyanidins.48,50,51 Importantly, procyanidins adsorb onto Caco-2 cell monolayers without affecting paracellular or transcellular transport.48 The magnitude of procyanidin protective effects against DCA-induced cell damage increases with the number of monomer units forming the procyanidin.48 These findings are in line with our previous results in liposomes, stressing the relevance of procyanidin–membrane interactions in the protection of the intestinal barrier against mechanical damage.
Bile acids promote an increase in paracellular transport by compromising the integrity of the tight junctions.52 DCA induced the redistribution of the protein ZO-153 from the tight junctions towards the cytosol, which was associated with an increase in paracellular transport.48 Both effects were prevented when cells were treated with the hexamer prior to the exposure to DCA.48 The maintenance of the integrity of the apical plasma membrane of intestinal cells, as well as the preservation of cell tight junctions, is critical for the correct functioning of the epithelial barrier. It has been reported that in both Caco-2 cells and normal human intestinal cells, tight junctions determine two kinds of hydrophilic pores, the smaller ones with an estimated radii of 5–6 Å and the larger ones with a radii >10 Å.54 Thus, tight junction pores limit the size of the molecules that can cross the epithelium, and only small water-soluble molecules are allowed to permeate through them.55,56 In the case of tight junction pores being altered and paracellular transport being increased, not only external large, toxic macromolecules but also potentially pathogenic microorganisms may penetrate the intestinal epithelium, initiate a cycle of local inflammation and/or reach the bloodstream and trigger inflammation and damage in other tissues.57,58
Another undesired effect of bile acids on intestinal cells is the promotion of intracellular oxidant generation. For example, the treatment of the isolated crypt epithelium with DCA or of Caco-2 cells with cholic acid stimulated the production of oxidants.52,59 The effect of DCA in promoting oxidant generation in Caco-2 cells was in part due to the activation of the enzyme NADPH oxidase,48,52 which is one of the main sources of cellular superoxide anions. We found that DCA-mediated generation of oxidants could be prevented by the hexamer in a concentration-dependent manner.48 Accordingly, the hexamer also inhibited the NADPH-dependent increase in oxidants triggered by the pro-inflammatory cytokine tumor necrosis factor alpha (TNFα).60
The higher oxidant production contributes to the DCA-mediated increase of Caco-2 cell barrier permeability.48 In humans and in experimental animals the permeabilization of the intestinal barrier can lead to disease. In this regard, the loss of intestinal barrier integrity constitutes a major pathological event proposed to underlie the development and/or progression of several human diseases including inflammatory bowel diseases (e.g. ulcerative colitis and Crohn's disease),61–64 alcoholic liver disease,65 obesity-triggered cardiovascular disease,66 insulin resistance and type 2 diabetes,67 coeliac disease68 and food allergies in general,69 among others. Therefore, procyanidins being able to protect intestinal cells from the deleterious effects of select damaging compounds (e.g. DCA, TNFα),60 the regular consumption of procyanidin-rich foods may alleviate the symptoms of those intestinal pathologies where the integrity of the intestinal barrier is compromised. Very importantly, given the relevance of the microbiota on intestinal permeability and endotoxin production, the potential effects of procyanidins on intestinal microbiota can also be a major aspect of the beneficial health effects of procyanidins on gastrointestinal health.
Caco-2 cells and the colonic mucosa express high levels of the isoform NADPH oxidase-1, a superoxide anion generating enzyme whose activation has been related to colonic inflammation and oncogenesis.70 This enzyme is located at the plasma membrane and co-localizes with caveolin, which is a characteristic lipid-raft protein.70,71 The mechanism of DCA-mediated NADPH oxidase activation is still under elucidation. Mello-Vieira et al. recently demonstrated that DCA incorporates into lipid rafts and alters their dynamics,51 an effect that explains the activation of certain lipid raft-associated enzymes observed in DCA-treated cells. In addition, it was demonstrated that the exposure of HCT116 cells to DCA causes the activation of the receptor of the epidermal growth factor (EGF) even in the absence of its ligand.72 EGF receptor resides in lipid rafts, and its activation leads to the downstream activation of the mitogen-activated protein kinases (MAPKs) ERK1/2, p38 and JNK all participants in bile salt-induced oncogenesis73,74 and apoptosis.75 In addition, activation of these signaling cascades promotes transcription of oncogenes and cell proliferation, and are found to be activated in colorectal cancer.76–78 We observed that Caco-2 cell treatment with DCA markedly activated p38, ERK1/2 and Akt (PKB) with an associated increase in intracellular calcium.50 All these events were prevented by the hexamer in a concentration-dependent manner.50 Supporting potential anticancer actions of procyanidins the regular consumption of food rich in these compounds has been associated with decreased risks for colorectal cancer.79
Interactions of procyanidins with lipid rafts
We next investigated whether specific interactions of the hexamer with lipid rafts were involved in the protective effects of this procyanidin. First, we evaluated the impact of the hexamer on the Caco-2 cell membrane fluidity as a parameter for the procyanidin interaction with the membrane. The hexamer decreased the fluidity of the cell apical portion of the plasma membrane, but the effect was restricted to the most superficial level of the membrane, close to the lipid polar headgroups.33 The removal of cholesterol from the membrane with methyl-β-cyclodextrin80 prevented the rigidifying effect of the hexamer, and provided additional evidence of cholesterol participation in hexamer binding to membranes.33 Subsequent cell treatment with DCA had a minor effect on hexamer-mediated membrane rigidification. To corroborate the involvement of cholesterol in hexamer-Caco-2 cell membrane interactions, cholesterol distribution in the plasma membrane was evaluated. Cholesterol showed a characteristic punctuated distribution in the plasma membrane that indicates its presence in discrete domains (lipid rafts). These domains were not affected by the hexamer neither in size nor in the pattern of membrane distribution.33 The diffuse localization of cholesterol in DCA-treated cells indicates an alteration in cholesterol distribution, similar to that observed for methyl-β-cyclodextrin.33 Interestingly, when cells were first incubated with the hexamer and subsequently exposed to DCA, the pattern of cholesterol distribution was preserved33 suggesting that DCA could not reach the same regions of the membrane when the hexamer was present. Similarly, cell pre-incubation with the hexamer prevented cholesterol removal by methyl-β-cyclodextrin. Resembling findings in liposomes, these results suggest that the hexamer interacts with cholesterol and prevents its redistribution and/or removal by external agents. Furthermore, and provided that the punctuated labeling of cholesterol showed lipid raft distribution, the capacity of the hexamer to prevent its redistribution by DCA indicates that this procyanidin interacts with lipid raft components, an effect that explains its ability to prevent the activation of lipid raft-associated cell signals50,60 (Fig. 4).In summary, we obtained evidence indicating that the hexamer, as a model of large procyanidins, can (a) prevent DCA-mediated deleterious effects in Caco-2 cells, most of them occurring in the lipid raft environment;48,50 (b) interact with membrane lipids and protect them preserving the integrity of the membrane upon DCA treatment;21,33 and (c) interact preferentially with zones of the membranes containing cholesterol, a key component of lipid rafts.33 It is important to stress that the membrane-related effects of procyanidins discussed in this section can be physiologically relevant. The effective concentrations of the hexamer are in the low micromolar range, e.g. 10 μM, which are concentrations expected in the gastrointestinal tract upon the consumption of procyanidin-rich foods or beverages.81
Concluding remarks
Ever since Renaud and Lorgeril suggested red wine consumption as an explanation for the “French paradox”,82 the incorporation of flavonoid-rich foods and beverages into the diet, as well as pharmacological supplementation with these compounds, has been associated with improvements in health. However, there is still limited information on the molecular mechanisms behind the impact of flavonoids on health. This also implies the need to identify the actual compounds acting on the biological system. The complete elucidation of how and where flavonoids may act is an enormous challenge given that they include hundreds of individual molecules that, once ingested and metabolized, render metabolites with still not fully known biological activities.Over the last decade, our group was interested in elucidating the mechanisms by which flavan-3-ols and procyanidins may act. Monomeric flavan-3-ols and, to a lesser extent, their dimers can enter cells and exert systemic biological effects. On the other hand, being larger molecules, the procyanidins cannot be absorbed and their presence is mostly restricted to the gastrointestinal tract.6,40 Therefore, effects of procyanidins occur principally in the gastrointestinal lumen in part via their interaction with the exofacial side of the enterocyte apical membrane. We have presented extensive evidence demonstrating that procyanidins have a key role in stabilizing membranes, preventing their disruption by chemical and biological agents, and regulating membrane-associated events. In this regard, procyanidins mitigate oxidative stress, the activation of proinflammatory and oncogenic signals, and the permeabilization of the intestinal epithelial barrier. Procyanidins may, therefore, help preserve the function and integrity of the intestinal epithelium, and thereby impact favorably on gastrointestinal health.
In summary the chemical and physical interactions of flavan-3-ols and procyanidins with cell membranes provide explanations consistent with their molecular structure, and tissue presence, based on experimental evidence obtained using synthetic membranes, cell cultures, and animal models. Future studies should be addressed to corroborate the involvement of the proposed mechanisms in the ability of flavan-3-ols and procyanidins to prevent/ameliorate diseases that directly or indirectly involve a loss of intestinal barrier integrity. This prospective research should consider that intact flavan-3-ols and procyanidins could exert other beneficial effects at the gastrointestinal tract such as inhibition of digestive enzymes, changing the microbiota population, and/or direct antioxidant actions. Furthermore, flavan-3-ols and procyanidins can be metabolized by the microbiota to smaller phenolic compounds, which increases the number of molecules potentially responsible for their beneficial effects on health.
Abbreviations
| AAPH | 2,2′-Azobis (2-amidinopropane) hydrochloride; |
| aGM1 | Asialo ganglioside GM1; |
| AMVN | 2,2′-Azobis (2,4-dimethylvaleronitrile); |
| DCA | Sodium deoxycholate; |
| DOPC | Dioleoyl phosphatidylcholine; |
| DPPC | Dipalmitoyl phosphatidylcholine; |
| EGCG | Epigallocatechin gallate; |
| EGF | Epidermal growth factor; |
| MAPK | Mitogen-activated protein kinase; |
| PC | Phosphatidylcholine; |
| POPC | 1-Palmitoyl 2-oleoyl phosphatidylcholine; |
| PS | Phosphatidylserine; |
| SM | Sphingomyelin |
Acknowledgements
This work was supported by grants from NIFA-USDA (CA-D*-xxx-7244-H) and the University of California, Davis, from UBACyT (20020120100177), CONICET (PIP 20110100752) and ANPCyT (PICT 2012-0765). SVV and CGF are members and PIO is an honorary member from CONICET, Argentina.References
- C. G. Fraga and P. I. Oteiza, Free Radicals Biol. Med., 2011, 51, 813–823 CrossRef CAS PubMed.
- J. K. Hellstrom, A. R. Torronen and P. H. Mattila, J. Agric. Food Chem., 2009, 57, 7899–7906 CrossRef CAS PubMed.
- V. Knaze, R. Zamora-Ros, L. Lujan-Barroso, I. Romieu, A. Scalbert, N. Slimani, E. Riboli, C. T. van Rossum, H. B. Bueno-de-Mesquita, A. Trichopoulou, V. Dilis, K. Tsiotas, G. Skeie, D. Engeset, J. R. Quiros, E. Molina, J. M. Huerta, F. Crowe, E. Wirfal, U. Ericson, P. H. Peeters, R. Kaaks, B. Teucher, G. Johansson, I. Johansson, R. Tumino, H. Boeing, D. Drogan, P. Amiano, A. Mattiello, K. T. Khaw, R. Luben, V. Krogh, E. Ardanaz, C. Sacerdote, S. Salvini, K. Overvad, A. Tjonneland, A. Olsen, M. C. Boutron-Ruault, G. Fagherazzi, F. Perquier and C. A. Gonzalez, Br. J. Nutr., 2012, 108, 1095–1108 CrossRef CAS PubMed.
- L. Gu, M. A. Kelm, J. F. Hammerstone, G. Beecher, J. Holden, D. Haytowitz, S. Gebhardt and R. L. Prior, J. Nutr., 2004, 134, 613–617 CAS.
- L. J. Porter, Z. Ma and B. G. Chan, Phytochemistry, 1991, 30, 1657–1663 CrossRef CAS.
- Y. Y. Choy, G. K. Jaggers, P. I. Oteiza and A. L. Waterhouse, J. Agric. Food Chem., 2012, 61, 121–127 CrossRef PubMed.
- J. J. Karchesy and R. W. Hemingway, J. Agric. Food Chem., 1986, 34, 966–970 CrossRef CAS.
- J. Wollgast and E. Anklam, Food Res. Int., 2000, 33, 423–447 CrossRef CAS.
- J. Yu, M. Ahmedna, I. Goktepe and J. Dai, J. Food Comp. Anal., 2006, 19, 364–371 CrossRef CAS PubMed.
- M. Galleano, S. V. Verstraeten, P. I. Oteiza and C. G. Fraga, Arch. Biochem. Biophys., 2010, 501, 23–30 CrossRef CAS PubMed.
- L. Porter, in Plant Polyphenols, ed. R. Hemingway and P. Laks, Springer US, 1992, vol. 59, ch. 14, pp. 245–258 Search PubMed.
- R. A. Dixon, D. Y. Xie and S. B. Sharma, New Phytol., 2005, 165, 9–28 CrossRef CAS PubMed.
- I. Hernandez, L. Alegre, F. Van Breusegem and S. Munne-Bosch, Trends Plant Sci., 2009, 14, 125–132 CrossRef CAS PubMed.
- E. Haslam, Phytochemistry, 1977, 16, 1625–1640 CrossRef CAS.
- G. G. Mackenzie, J. M. Delfino, C. L. Keen, C. G. Fraga and P. I. Oteiza, Biochem. Pharmacol., 2009, 78, 1252–1262 CrossRef CAS PubMed.
- S. V. Verstraeten, J. F. Hammerstone, C. L. Keen, C. G. Fraga and P. I. Oteiza, J. Agric. Food Chem., 2005, 53, 5041–5048 CrossRef CAS PubMed.
- A. G. Erlejman, S. V. Verstraeten, C. G. Fraga and P. I. Oteiza, Free Radicals Res., 2004, 38, 1311–1320 CrossRef CAS PubMed.
- I. Pianet, Y. André, M.-A. Ducasse, I. Tarascou, J.-C. Lartigue, N. l. Pinaud, E. Fouquet, E. J. Dufourc and M. Laguerre, Langmuir, 2008, 24, 11027–11035 CrossRef CAS PubMed.
- J. F. Hammerstone, S. A. Lazarus and H. H. Schmitz, J. Nutr., 2000, 130, 2086S–2092S CAS.
- W. Bors, W. Heller, C. Michel and M. Saran, Methods Enzymol., 1990, 186, 343–355 CAS.
- S. V. Verstraeten, C. L. Keen, H. H. Schmitz, C. G. Fraga and P. I. Oteiza, Free Radicals Biol. Med., 2003, 34, 84–92 CrossRef CAS.
- S. B. Lotito, L. Actis-Goretta, M. L. Renart, M. Caligiuri, D. Rein, H. H. Schmitz, F. M. Steinberg, C. L. Keen and C. G. Fraga, Biochem. Biophys. Res. Commun., 2000, 276, 945–951 CrossRef CAS PubMed.
- E. Niki, in Methods Enzymol., ed. L. Packer and A. Glazer, Academic Press, 1990, vol. 186, pp. 100–108 Search PubMed.
- J. L. Browning, Biochemistry, 1981, 20, 7144–7151 CrossRef CAS.
- R. D. Kaiser and E. London, Biochemistry, 1999, 38, 2610 CrossRef CAS PubMed.
- H. Tsuchiya, Chem. – Biol. Interact., 2001, 134, 41–54 CrossRef CAS.
- N. Poklar Ulrih, A. Ota, M. Šentjurc, S. Kure and V. Abram, Food Chem., 2010, 121, 78–84 CrossRef CAS PubMed.
- T. W. Sirk, E. F. Brown, M. Friedman and A. K. Sum, J. Agric. Food Chem., 2009, 57, 6720–6728 CrossRef CAS PubMed.
- I. Tarascou, M. A. Ducasse, E. J. Dufourc, D. Moskau, E. Fouquet, M. Laguerre and I. Pianet, Magn. Reson. Chem., 2007, 45, 157–166 CrossRef CAS PubMed.
- X. Yu, S. Chu, A. E. Hagerman and G. A. Lorigan, J. Agric. Food Chem., 2011, 59, 6783–6789 CrossRef CAS PubMed.
- A. Domecq, E. A. Disalvo, D. L. Bernik, F. Florenzano and M. J. Politi, Drug Delivery, 2001, 8, 155–160 CrossRef CAS PubMed.
- T. A. Brasitus and D. Schachter, Biochemistry, 1980, 19, 2763–2769 CrossRef CAS.
- S. V. Verstraeten, G. K. Jaggers, C. G. Fraga and P. I. Oteiza, Biochim. Biophys. Acta, Biomembr., 2013, 1828, 2646–2653 CrossRef CAS PubMed.
- R. Y. Patel and P. V. Balaji, J. Phys. Chem. B, 2008, 112, 3346–3356 CrossRef CAS PubMed.
- J. H. Ipsen, G. Karlstrom, O. G. Mouritsen, H. Wennerstrom and M. J. Zuckermann, Biochim. Biophys. Acta, 1987, 905, 162–172 CrossRef CAS.
- B. R. Lentz, D. A. Barrow and M. Hoechli, Biochemistry, 1980, 19, 1943–1954 CrossRef CAS.
- O. G. Mouritsen and M. J. Zuckermann, Lipids, 2004, 39, 1101–1113 CrossRef CAS PubMed.
- J. Zidar, F. Merzel, M. Hodoscek, K. Rebolj, K. Sepcic, P. Macek and D. Janezic, J. Phys. Chem. B, 2009, 113, 15795–15802 CrossRef CAS PubMed.
- S. N. Ahmed, D. A. Brown and E. London, Biochemistry, 1997, 36, 10944–10953 CrossRef CAS PubMed.
- C. Tsang, C. Auger, W. Mullen, A. Bornet, J. M. Rouanet, A. Crozier and P. L. Teissedre, Br. J. Nutr., 2005, 94, 170–181 CrossRef CAS.
- C. Manach, G. Williamson, C. Morand, A. Scalbert and C. Remesy, Am. J. Clin. Nutr., 2005, 81, 230S–242S CAS.
- A. Sano, J. Yamakoshi, S. Tokutake, K. Tobe, Y. Kubota and M. Kikuchi, Biosci., Biotechnol., Biochem., 2003, 67, 1140–1143 CrossRef CAS PubMed.
- S. Deprez, I. Mila, J. F. Huneau, D. Tome and A. Scalbert, Antioxid. Redox Signaling, 2001, 3, 957–967 CrossRef CAS PubMed.
- Y. Y. Choy, G. K. Jaggers, P. I. Oteiza and A. L. Waterhouse, J. Agric. Food Chem., 2013, 61, 121–127 CrossRef CAS PubMed.
- I. J. Hidalgo, T. J. Raub and R. T. Borchardt, Gastroenterology, 1989, 96, 736–749 CAS.
- M. D. Peterson and M. S. Mooseker, J. Cell Sci., 1993, 105(Pt 2), 445–460 Search PubMed.
- V. Meunier, M. Bourrié, Y. Berger and G. Fabre, Cell Biol. Toxicol., 1995, 11, 187–194 CrossRef CAS.
- A. G. Erlejman, C. G. Fraga and P. I. Oteiza, Free Radicals Biol. Med., 2006, 41, 1247–1256 CrossRef CAS PubMed.
- P. R. Debruyne, E. A. Bruyneel, X. Li, A. Zimber, C. Gespach and M. M. Mareel, Mutat. Res., 2001, 480–481, 359–369 CrossRef CAS.
- M. Da Silva, G. K. Jaggers, S. V. Verstraeten, A. G. Erlejman, C. G. Fraga and P. I. Oteiza, Free Radicals Biol. Med., 2012, 52, 151–159 CrossRef CAS PubMed.
- J. Mello-Vieira, T. Sousa, A. Coutinho, A. Fedorov, S. D. Lucas, R. Moreira, R. E. Castro, C. M. Rodrigues, M. Prieto and F. Fernandes, Biochim. Biophys. Acta, 2013, 1828, 2152–2163 CrossRef CAS PubMed.
- Y. Araki, T. Katoh, A. Ogawa, S. Bamba, A. Andoh, S. Koyama, Y. Fujiyama and T. Bamba, Free Radicals Biol. Med., 2005, 39, 769–780 CrossRef CAS PubMed.
- C. M. Van Itallie, A. S. Fanning, A. Bridges and J. M. Anderson, Mol. Biol. Cell, 2009, 20, 3930–3940 CrossRef CAS PubMed.
- J. Linnankoski, J. Makela, J. Palmgren, T. Mauriala, C. Vedin, A. L. Ungell, L. Lazorova, P. Artursson, A. Urtti and M. Yliperttula, J. Pharm. Sci., 2010, 99, 2166–2175 CAS.
- S. D. Flanagan, L. H. Takahashi, X. Liu and L. Z. Benet, J. Pharm. Sci., 2002, 91, 1169–1177 CrossRef CAS PubMed.
- K. Lee and D. R. Thakker, J. Pharm. Sci., 1999, 88, 680–687 CrossRef CAS PubMed.
- J. L. Madara, J. Clin. Invest., 1989, 83, 1089–1094 CrossRef CAS PubMed.
- C. J. Watson, M. Rowland and G. Warhurst, Am. J. Physiol. Cell Physiol., 2001, 281, C388–C397 CAS.
- P. A. Craven, J. Pfanstiel, R. Saito and F. R. DeRubertis, Cancer Res., 1986, 46, 5754–5759 CAS.
- A. G. Erlejman, G. Jaggers, C. G. Fraga and P. I. Oteiza, Arch. Biochem. Biophys., 2008, 476, 186–195 CrossRef CAS PubMed.
- A. Keshavarzian, A. Banan, A. Farhadi, S. Komanduri, E. Mutlu, Y. Zhang and J. Z. Fields, Gut, 2003, 52, 720–728 CrossRef CAS.
- S. J. McKenzie, M. S. Baker, G. D. Buffinton and W. F. Doe, J. Clin. Invest., 1996, 98, 136–141 CrossRef CAS PubMed.
- H. Kimura, R. Hokari, S. Miura, T. Shigematsu, M. Hirokawa, Y. Akiba, I. Kurose, H. Higuchi, H. Fujimori, Y. Tsuzuki, H. Serizawa and H. Ishii, Gut, 1998, 42, 180–187 CrossRef CAS.
- J. Pravda, World J. Gastroenterol., 2005, 11, 2371–2384 CAS.
- R. Rao, Hepatology, 2009, 50, 638–644 CrossRef CAS PubMed.
- A. L. Neves, J. Coelho, L. Couto, A. Leite-Moreira and R. Roncon-Albuquerque, Jr., J. Mol. Endocrinol., 2013, 51, R51–R64 CrossRef CAS PubMed.
- F. Horton, J. Wright, L. Smith, P. J. Hinton and M. D. Robertson, Diabet. Med., 2014, 31, 559–563 CrossRef CAS PubMed.
- M. Heyman, J. Abed, C. Lebreton and N. Cerf-Bensussan, Gut, 2012, 61, 1355–1364 CrossRef CAS PubMed.
- C. Perrier and B. Corthesy, Clin. Exp. Allergy, 2011, 41, 20–28 CrossRef CAS PubMed.
- K. Rokutan, T. Kawahara, Y. Kuwano, K. Tominaga, K. Nishida and S. Teshima-Kondo, Semin. Immunopathol., 2008, 30, 315–327 CrossRef CAS PubMed.
- I. Helmcke, S. Heumuller, R. Tikkanen, K. Schroder and R. P. Brandes, Antioxid. Redox Signaling, 2009, 11, 1279–1287 CrossRef CAS PubMed.
- S. Jean-Louis, S. Akare, M. A. Ali, E. A. Mash, Jr., E. Meuillet and J. D. Martinez, J. Biol. Chem., 2006, 281, 14948–14960 CrossRef CAS PubMed.
- H. Y. Lee, S. Crawley, R. Hokari, S. Kwon and Y. S. Kim, Int. J. Oncol., 2010, 36, 941–953 CAS.
- K. Allen, N. D. Kim, J. O. Moon and B. L. Copple, Toxicol. Appl. Pharmacol., 2010, 243, 63–67 CrossRef CAS PubMed.
- D. Qiao, E. D. Stratagouleas and J. D. Martinez, Carcinogenesis, 2001, 22, 35–41 CrossRef CAS.
- J. Y. Fang and B. C. Richardson, Lancet Oncol., 2005, 6, 322–327 CrossRef CAS.
- M. W. Saif, Expert Opin. Invest. Drugs, 2010, 19, 357–369 CrossRef CAS PubMed.
- M. W. Saif and E. Chu, Cancer J., 2010, 16, 196–201 CrossRef CAS PubMed.
- M. Rossi, E. Negri, M. Parpinel, P. Lagiou, C. Bosetti, R. Talamini, M. Montella, A. Giacosa, S. Franceschi and C. La Vecchia, Cancer, Causes Control, 2010, 21, 243–250 CrossRef PubMed.
- S. K. Rodal, G. Skretting, O. Garred, F. Vilhardt, B. van Deurs and K. Sandvig, Mol. Biol. Cell., 1999, 10, 961–974 CrossRef CAS.
- L. Y. Rios, R. N. Bennett, S. A. Lazarus, C. Remesy, A. Scalbert and G. Williamson, Am. J. Clin. Nutr., 2002, 76, 1106–1110 CAS.
- S. Renaud and M. de Lorgeril, Lancet, 1992, 339, 1523–1526 CrossRef CAS.
- A. W. Schuttelkopf and D. M. van Aalten, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2004, 60, 1355–1363 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2015 |