Novel polymer nanocomposites from bioinspired green aqueous functionalization of BNNTs†
Vijay Kumar
Thakur
a,
Jian
Yan
a,
Meng-Fang
Lin
b,
Chunyi
Zhi
c,
Dmitri
Golberg
c,
Yoshio
Bando
c,
Raymond
Sim
b and
Pooi See
Lee
*ab
aTemasek Laboratories@NTU, Research Techno Plaza, BorderX Block, 50 Nanyang Drive, Singapore 637553. E-mail: pslee@ntu.edu.sg
bSchool of Materials Science and Engineering, Nanyang Technological University, 50 Nanyang Avenue, Singapore 639798
cInternational Centre for Materials Nanoarchitectonics (MANA), National Institute for Materials Science (NIMS), Namiki 1-1, Tsukuba, Ibaraki 305-0044, Japan
First published on 31st January 2012
Abstract
Boron nitride nanotubes (BNNTs) are excellent nanofillers to enhance the mechanical and thermal properties of polymer nanocomposites. Despite the rapid progress in the effective syntheses of BNNTs, the ease of processability and solubility are major roadblocks for their widespread applications. The present work reports for the first time: a facile and environment-friendly green approach for aqueous bioinspired functionalization of boron nitride nanotubes through the use of dopamine, a synthetic mimic of mussel adhesive proteins. This approach is based on the affinity of the amino group of dopamine molecules for the boron atoms in BNNTs and π–π interactions as well as van der Waals interactions between the BNNTs network and dopamine molecules. Functionalization of the boron nitride nanotubes was evidently found to be associated with the existing π–π bonding and van der Waals interactions at the BNNT surfaces, involving the aromatic core structures of the dopamine molecules based on various characterizations. The resultant functionalized BNNTs are highly dispersible in water and a number of solvents. Polymer nanocomposites were prepared using pristine and dopamine functionalized BNNTs as reinforcement in a poly(vinyl difluoroethylene) matrix and tested for the thermal and mechanical properties. The functionalized BNNT reinforced polymer nanocomposites exhibit superior properties as compared to nanocomposites based on pristine BNNTs. This comprehensive study indicates that dopamine functionalized BNNTs are promising materials for various applications and are expected to form the basis of a new class of chemically reactive nanostructures.
Introduction
Among the emerging nanomaterials, boron and its related nanostructures have become more fascinating because of their unique structures and extraordinary properties.1 Bulk boron nitride (BN) is a non-oxide ceramic, and has been commonly used in industrial applications due to its unique physical, chemical and electronic properties. Boron nitride nanotubes (BNNTs), having a structure similar to the carbon nanotubes (CNT), were first synthesized in 1995.2 Since then a number of synthesis methods have been used to grow BNNTs, such as arc discharge, laser heating, carbon nanotube based substitution reaction and chemical vapor deposition (CVD).3–5 Boron nitride nanotubes (BNNTs), consisting of equal proportions of boron and nitrogen atoms, are made of rolled-up hexagonal sheets. The length of a boron nitride nanotube can be greater than one micrometre and the diameter is on the order of nanometres. Along the radial direction there may be one or more layers of boron nitride sheets and these sheets are called single walled and multi-walled boron nitride nanotubes respectively. BNNTs exhibit excellent mechanical, electrical and thermal properties.6 BNNTs have been found to be more resistant to oxidation as compared to their counterpart CNTs and hence can be used in high temperature applications.7 These single-walled and multiwalled BNNTs exhibit nearly constant band gaps independent of the chirality of BNNTs and the tube diameter. The properties of BNNTs are in sharp contrast to those of carbon nanotubes (CNTs) whose electronic properties are dependent on the chiral index (n, m).8,9 Indeed, their unique electronic properties associated with thermal and chemical stabilities and mechanical toughness render BNNTs a promising medium for applications in field emission, nano-electronics and optoelectronics, protective tubular shields, nanotransistors, and gas sensors in extreme environments. Because of their large surface-to-mass ratio and their potential applications in miniaturized chemical sensors, adsorption properties of BNNTs have also received considerable attention. Unfortunately, the poor solubility, inherent low chemical reactivity of the surface of well-crystallized BNNTs and difficulties of purifying and processing have hampered the rapid application of BNNTs.10,11 Functionalization of BNNTs is considered one of the solutions to overcome these problems.12–14 However, as compared to CNTs where several functionalization schemes exist, limited research has been reported on the functionalization of BNNTs.15,16 So far, BNNTs have been modified through covalent and noncovalent interactions.17–20 However, the absence of an easy and effective method to achieve an elevated concentration of functional groups on BNNTs still poses a roadblock to the successful modification of chemically active BNNTs. Development of simple methods for aqueous functionalization of BNNTs has proven challenging and only a few methods of accomplishing this have been previously reported.21–24Our approach is inspired by a fascinating adhesive protein molecule, dopamine.25–27 Dopamine is believed to interact strongly with a number of materials such as polymers, metals and metal oxides.25 Herein, we describe the successful green aqueous functionalization of BNNTs with amine functional groups of dopamine, giving the strong π–π as well as van der Waals interactions and their application as reinforcement in poly(vinylidene fluoride) (PVDF) polymer for novel nanocomposites.5,6,12,21,24 PVDF was chosen as the polymer matrix in the present work due to its remarkable properties such as high stability, chemical inertness, high volume resistivity, excellent thermal stability, low water absorption rate, and low shrinking rate. Furthermore excellent mechanical properties, biocompatibility, relatively low cost, easy processing, flexibility and light weight make it a potential candidate in an enormous range of applications such as from electrical components to tactile sensor arrays, from biomedical to energy storage devices, etc.25–28 The extra functional groups were generated at the surface of the BNNTs using a simple reflux treatment of BNNTs. In contrast to the parent BNNTs, the functionalized BNNTs are soluble or dispersible in common organic solvents such as N,N-dimethylformamide, chloroform, N,N-dimethylacetamide and ethanol. Bioinspired functionalization of nanotubes through dopamine offers an effective route to enhance the solubility of nanotubes in solvents, thereby deterring nanotubes from association and clustering. Furthermore, the novel nanocomposites prepared using functionalized nanotubes exhibit promising thermal and mechanical properties.
Experimental section
Materials
The following materials were used in this study: dopamine hydrochloride (Sigma-Aldrich), boron nitride nanotubes (BNNTs) and 2-amino-2-hydroxymethylpropane-1,3-diol (Tris, Aldrich). Ultrapure water (resistivity = 18.2 MΩ) was used in the whole experiment. Poly(vinyl difluoroethylene) (PVDF) polymer samples used in this work were in powder form having a diameter of 0.1 mm (Solvay Solexis Inc). Solvents N,N-dimethylformamide, chloroform, N,N-dimethylacetamide and ethanol were supplied by Sigma-Aldrich Chemie GmbH, Germany.Synthesis of boron nitride nanotubes (BNNTs)
High-purity multiwalled BNNTs were synthesized by a chemical vapor deposition (CVD) method.6 In brief, a mixture of boron, SnO and MgO in an appropriate ratio placed in a BN-made reaction tube was heated to 1500 °C. The heating was carried out using an RF inducting furnace. Such a high temperature allows the reaction of boron with MgO to form B2O2 and Mg vapor. Subsequently the vapours were argon-transported into a reaction chamber maintained at a temperature of 1300 °C followed by the introduction of a flow of ammonia. The chemical reaction between B2O2 and ammonia results in the formation of BNNTs which can be collected from the BN wall of the chamber by fully evaporating the reaction mixture.Functionalization of boron nitride nanotubes (BNNTs)
50 mg of dopamine hydrochloride was dissolved in 200 mL of 10 mM Tris–Cl aqueous solution (pH = 8.5) by sonication for 30 minutes in an ice bath. 50 mg of BNNTs were added to this solution and again dispersed by sonication for 10 min in an ice bath. Subsequently the surface chemical modification was performed at 50 °C for seventy-two hours under vigorous stirring in a heating mantle fitted with a reflux condenser which was sealed. The solution's colour changed to dark brown due to pH-induced oxidation. After the completion of the reaction, the polydopamine functionalized BNNTs (BNNTs@PDOPA) were filtered with a 0.02 μm membrane filter, followed by an entire washing with deionized water for several times. The functionalized BNNTs recovered by filtration were dried under reduced pressure for 24 h.Preparation of PVDF/BNNT composites
In order to prepare PVDF/(pristine-BNNT or functionalized-BNNT) nanocomposites, 10 mg BNNTs or dopamine functionalized BNNTs were mixed with 17 mL of N,N-dimethylformamide. The mixture was magnetically stirred for 7 hours followed by sonication for 120 minutes resulting in BNNTs solution. Subsequently, 990 mg PVDF was dissolved in the solution. This solution was further stirred for six hours at 60 °C followed by sonication. To prepare the nanocomposite films of uniform thickness, a customized laboratory-scale tape caster was used. The nanocomposites solution was cast onto a glass carrier and dried in air at 60 °C for 5 hours and in a vacuum at 50 °C for 12 hours to remove DMF. The thickness of the tape was measured using a micrometre scale accurate to 0.001 mm; the weight of the samples was measured using a laboratory scale accurate to 0.01 mg.Characterization
The functionalized BNNTs were characterized by Fourier transform infrared (FTIR) spectroscopy, Raman spectroscopy, thermogravimetric analysis (TGA), FESEM and TEM (see the corresponding descriptions in the ESI†). Tensile tests to determine the tensile strength and modulus were performed on specimens cut from the above fabricated polymer and nanocomposite films. The measurements were made on a computerized testing machine (Instron, Canton, MA). The specimens of dimension 15 mm × 3 mm × 0.025 mm were used for analysis. The tensile tests were conducted in accordance with the ASTM D 638 method. Five specimens of each sample were used for the measurement of the above mechanical properties at ambient laboratory environment at a deformation rate of 5 mm min−1 and the values reported were averages of five measurements. The coefficients of thermal expansion (CTEs) of the pristine PVDF and the nanocomposites of PVDF/pristine-BNNTs and PVDF/functionalized BNNTs were determined using a dynamic mechanical analyzer (Perkin-Elmer TMA 7e) in nitrogen.Results and discussion
Boron nitride nanotube functionalization
As discussed earlier, BNNTs have high chemical stability and are difficult to functionalize. In order to find a simple route for aqueous functionalization of the BNNTs, we have resorted to the unique presence of the π-electronic structure of BNNTs as well as the aromatic structure of the biomolecule dopamine. Herein we have introduced the biomimetic surface chemistry inspired by the mussel adhesion mechanism to develop a simple, versatile technique for the coating of polydopamine onto BNNTs. BNNTs are expected to generate π-stacking with the benzenoid molecules because the nanotubes have a six-member ring structure that consists of alternating B and N atoms.5,12,24 When BNNTs are added to an aqueous solution of dopamine, significant adhesion could be expected as a result of strong π–π interactions as well as van der Waals interactions between the BNNTs and interaction between the amino groups of the dopamine molecule5,10,12,21,24,29–31 (Scheme 1).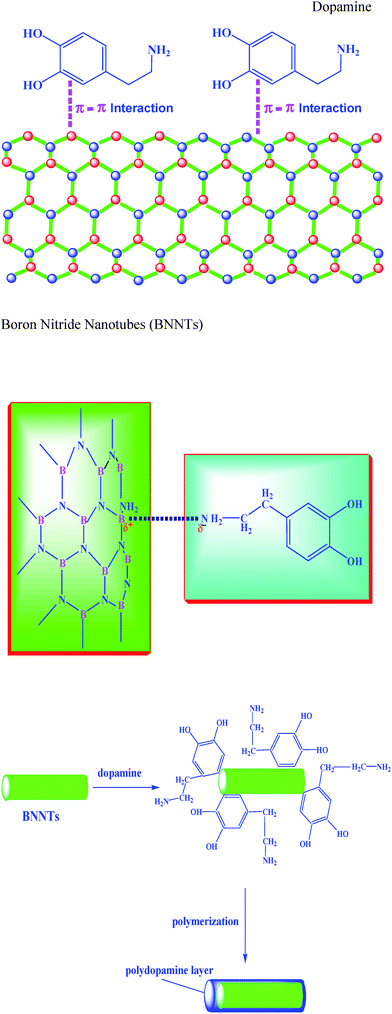 | ||
| Scheme 1 Schematic illustration of dopamine functionalization of BNNTs. | ||
The dopamine polymerization mechanism29–31 involves the oxidation of catechol in dopamine to quinone by alkaline pH-induced oxidation finally resulting in polydopamine (Scheme 2).
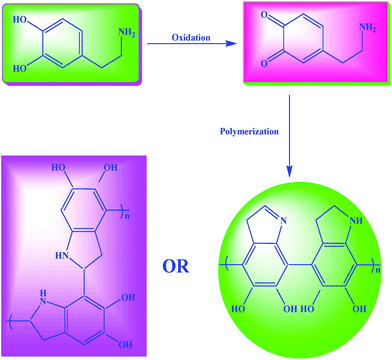 | ||
| Scheme 2 Polymerization of dopamine to polydopamine.29–31 | ||
Thin layers are formed on the surface through a simple deposition process with the oxidative self-polymerization of dopamine. Most researchers have reported that BNNTs are not dispersible in water even after functionalization. However, in our case the BNNTs after functionalization with polydopamine are thoroughly dispersed in the aqueous solutions and organic solvents compatible with polydopamine. We believe that the aromatic rings of dopamine promote strong π–π as well as van der Waals interactions with the BNNT surfaces.5,12,24,29–31 Furthermore, as evident from the structure of polydopamine it contains hydrophilic –OH and NH2 groups which also facilitate the interaction between BNNTs and H2O molecules, resulting in the dissolution of BNNTs in water. Fig. 1 shows the photographic images of pristine and functionalized BNNTs dispersed in water.
 | ||
| Fig. 1 Photographs of vials containing the pristine BNNTs (right-hand side) and the functionalized BNNTs (left-hand side) in water after standing overnight. | ||
It is clearly visible that pure BNNTs are colourless/white in colour and float in water referring to their insolubility in water, whereas the functionalized BNNTs display a dark brownish colour. The colour of modified nanotubes can be attributed to the self-polymerization of dopamine. The dopamine functionalities not only impart solubility to BNNTs but also make the nanotubes chemically active for further functionalization.
Characterization of the functionalized boron nitride nanotubes (BNNTs)
The presence of polydopamine coating on the surface of BNNTs was ascertained by different techniques (see the corresponding description in the ESI†). To confirm the functionalization and reveal the functionalization mechanism of BNNTs with dopamine, FTIR analysis was conducted on the pristine and functionalized BNNTs.In Fig. 2, the FTIR spectrum of pristine BNNTs exhibits two distinct peaks at 1370.0 cm−1 and 818.0 cm−1 which can be attributed to the in-plane B–N transverse optical mode of the BNNTs and the B–N–B out-of-plane bending vibration perpendicular to the axis of the nanotube respectively. The dopamine functionalized BNNTs also exhibit a spectrum similar to that of the pristine BNNTs. However, the surface-bound functional groups of dopamine which are strongly coupled with the nanotube walls dominate the IR spectrum of functionalized BNNTs. The absorptions corresponding to C–H bonds (bending vibrations: 1024, 1097 and 1261 cm−1, and stretching vibration at 2963 cm−1) can be clearly identified in the FTIR spectrum of the functionalized BNNTs, which indicates that dopamine chains are bonded to the BNNTs.29–32 The appearance of two vibrational modes at 1024 and 870 cm−1 are assigned to N–H out-of-plane bending absorptions of the amine group of dopamine (ESI, Fig. S1†). The phenolic C–O–H bending and stretching vibrations exist at 1369 and 1289 cm−1. The absorption peak at 1603 cm−1 is assigned to the overlap of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C resonance vibration in aromatic ring and N–H bending.29–32 The N–H shearing vibration of the amine group is observed at 1513 cm−1. The intense absorption features around 3600 cm−1 and 3100 cm−1 come from N–H/O–H stretching vibrations. Furthermore when we compare the intensity of the IR spectra of the functionalized BNNTs with that of pristine BNNTs, it is clearly visible that there is a significant decrease in the intensity of the 1370 cm−1 band. This again indicates the existence of interaction between the dopamine and the BNNTs. This effect can be associated with the π–π bonding and van der Waals interaction at the BNNT surfaces, involving the aromatic core structures of the dopamine molecules. Raman spectra of the pure BNNTs and polydopamine functionalized BNNTs (BNNTs@PDOPA) show significant differences. This indicates the strong interactions between pristine BNNTs and dopamine. As shown in Fig. 3, Raman spectra of both BNNTs and functionalized BNNTs showed the presence of the characteristic 1362 cm−1 band due to the E2g tangential mode. After the polydopamine coating, a new peak appeared at 1580 cm−1 (caused by the deformation of catechol), in addition to the intrinsic peaks of BNNTs. Furthermore the intensity of the BNNTs peak at 1362 cm−1 considerably decreased signifying a strong coating of polydopamine on BNNTs.
C resonance vibration in aromatic ring and N–H bending.29–32 The N–H shearing vibration of the amine group is observed at 1513 cm−1. The intense absorption features around 3600 cm−1 and 3100 cm−1 come from N–H/O–H stretching vibrations. Furthermore when we compare the intensity of the IR spectra of the functionalized BNNTs with that of pristine BNNTs, it is clearly visible that there is a significant decrease in the intensity of the 1370 cm−1 band. This again indicates the existence of interaction between the dopamine and the BNNTs. This effect can be associated with the π–π bonding and van der Waals interaction at the BNNT surfaces, involving the aromatic core structures of the dopamine molecules. Raman spectra of the pure BNNTs and polydopamine functionalized BNNTs (BNNTs@PDOPA) show significant differences. This indicates the strong interactions between pristine BNNTs and dopamine. As shown in Fig. 3, Raman spectra of both BNNTs and functionalized BNNTs showed the presence of the characteristic 1362 cm−1 band due to the E2g tangential mode. After the polydopamine coating, a new peak appeared at 1580 cm−1 (caused by the deformation of catechol), in addition to the intrinsic peaks of BNNTs. Furthermore the intensity of the BNNTs peak at 1362 cm−1 considerably decreased signifying a strong coating of polydopamine on BNNTs.
 | ||
| Fig. 2 Comparative FTIR spectra of pristine BNNTs and dopamine functionalized BNNTs. | ||
 | ||
| Fig. 3 Raman spectra of pristine BNNTs and dopamine functionalized BNNTs (BNNTs@PDOPA). | ||
Fig. 4 shows a typical X-ray diffraction (XRD) pattern of the functionalized BNNTs. The pattern clearly indicates that the material mainly consists of two phases: hexagonal BN and polydopamine.
The obviously broadened and reduced peaks of BNNTs suggest the appearance of amorphous polydopamine. As shown in the figure, BNNT@PDOPA exhibits only one of its parent peaks centered at 26.66° while the other characteristic peaks4,22 are missing meaning that the amorphous polymer dopamine has strongly covered the surface of the BNNTs. A broad diffraction peak of PDOPA (at 2θ = 12.38) has appeared indicating the successful functionalization of BNNTs.
The morphology of the functionalized BNNTs has been evaluated using the field emission scanning electron microscopy (FESEM) as shown in Fig. 5a and b. The amorphous and continuous surface of the functionalized BNNTs is significantly different from that of pure BNNTs.
 | ||
| Fig. 5 (a) FESEM image of pristine BNNTs. (b) FESEM image of dopamine functionalized BNNTs. | ||
The dopamine layers appear to be wrapping the BNNTs, associated with the strong π–π interactions as well as interaction of the amine group with the BNNT surfaces. More detailed studies have been carried out via transmission electron microscopy (TEM) which was further used to confirm the presence of dopamine onto the functionalized BNNTs. The result is shown in Fig. 6.
 | ||
| Fig. 6 TEM image of dopamine functionalized BNNTs (BNNTs@PDOPA). | ||
BNNTs@PDOPA in solution are well dispersed and retain a characteristic 1D tubular morphology. An amorphous layer can be observed on the BNNTs surface. All the BNNTs were shown to be homogeneously coated with uniform polydopamine layer, and the boundary is very clear. This confirms the presence of the dopamine as an amorphous surface layer on the coated boron nitride nanotubes.
With the results of FTIR, Raman Spectroscopy, XRD, FESEM and TEM, the amine and phenolic hydroxyl groups have been confirmed to adhere to the BNNTs surface via strong bonding. BNNTs could be introduced into homogeneous aqueous and organic solutions via the functionalization with dopamine molecules bearing amino and benzenoid moieties. A thermogravimetric analysis (TGA) was further carried out to investigate the weight of dopamine in the functionalized BNNTs (Fig. 7).
 | ||
| Fig. 7 TGA curves of dopamine, BNNTs and dopamine functionalized BNNTs (BNNTs@PDOPA). | ||
As shown in the figure, pristine BNNTs exhibit very high thermal stability and do not decompose up to 900 °C. The pure dopamine did not show any significant decomposition below approximately 220 °C, suggesting that the unbound dopamine possesses the essential thermal stability for the subsequent functionalization with suitable monomers for different applications. However, above 220 °C the dopamine exhibits significant thermal degradation which can be attributed to the degradation of the catechol moiety and the decomposition of the amine group. On the other hand the thermal degradation behavior of the BNNTs@PDOPA has been found to be qualitatively similar to that of the pure dopamine, although the degradation of the adsorbed polydopamine occurred at sufficiently higher temperatures. Thermogravimetric analyses of BNNTs@PDOPA revealed a steady mass loss between 400 and 900 °C which can be attributed to the weight loss of dopamine due to degradation of the dopamine molecules. The TGA results reveal that there is 20–25 wt% of dopamine in the functionalized BNNTs. This high content of dopamine in the BNNTs@PDOPA suggests that the functionalized BNNTs can be further modified with the desired monomer depending upon the requirement. These results further suggest that there is strong interaction between the dopamine moiety and BNNTs.
Polymeric nanocomposite based on functionalized BNNTs
As discussed earlier, BNNTs exhibit a number of unique physical properties which could be advantageous in polymeric nanocomposites. However, the poor dispersion of BNNTs in common solvents inhibits their use in a number of applications. The functionalized BNNTs provide means of improved BNNT–polymer matrix interfacial interactions. This may translate to enhanced mechanical properties as the mechanical load is expected to be transferred efficiently from the matrix to the BNNTs with its effective reinforcement properties or good interfacial bonding. In the present work we have used PVDF as a polymeric matrix due to its versatile applications. BNNT@PDOPA loaded nanocomposite polymer films were first studied by XRD, as shown in Fig. 8. | ||
| Fig. 8 Comparative XRD patterns of pristine PVDF polymer film and BNNTs@PDOPA/PVDF polymer nanocomposite film. | ||
We verified that BNNTs exist in the film from a characteristic sharp reflection at 2θ = 27° that corresponds to the hexagonal BN (002) plane spacing as shown in earlier section in the XRD spectrum of PDOPA@BNNTs. The other peaks present in the nanocomposite films can be easily assigned to that of the pristine PVDF polymer.27
The nanocomposites prepared using pristine and functionalized BNNTs were tested for tensile strength and thermomechanical analysis (TMA). Fig. 9 shows the tensile strength results of the pristine PVDF and nanocomposite films. It has been observed that the tensile modulus (Fig. 9a) of the pristine PVDF (1106 MPa) increases to a significant extent with the incorporation of pristine-BNNTs (1294 MPa) and dopamine functionalized BNNTs (1448 MPa). These results are in agreement with the previous results on nanocomposites based on polycarbonate (PC) and polyvinyl butyral (PVB) where 1 wt% fraction of pristine-BNNTs results in 10–20% increase in modulus and 1 wt% fraction of functionalized BNNTs results in 30–37% enhancement.33 Indeed the performance of the polymer nanocomposites is controlled by the properties of the matrix interaction and interfacial bonding. In the case of functionalized BNNT/PVDF matrix based nanocomposites, the better interaction between the PVDF polymer matrix and the functionalized BNNTs accounts for the enhanced mechanical properties as compared to pristine PVDF or pristine-BNNT/PVDF composites. The above results clearly indicate that the dopamine functionalization effectively improves the affinity of BNNTs for the polymer matrix. This, in turn, results in a more effective load transfer from the matrix to the nanotubes. The yield strength of the pristine PVDF has also been found to increase on the reinforcement of BNNTs (Fig. 9b). The dopamine functionalized BNNTs demonstrate better results as compared to pristine-BNNTs/PVDF, comparable to the previous results reported on hydroxylated BNNT nanocomposites.33
 | ||
| Fig. 9 Comparative tensile tests performed on PVDF, PVDF/BNNTs, PVDF/BNNT@PDOPA composites: (a) elastic modulus and (b) yield strength. | ||
The coefficient of thermal expansion (CTE) is another very important parameter in determining the dimensional stability of the polymer nanocomposites in a number of applications such as in packaging. Thermal mechanical analysis (TMA) was carried out, and the coefficients of thermal expansion (CTEs) of the pristine PVDF and the nanocomposite films with pristine and dopamine functionalized BNNTs (BNNTs@PDOPA) were compared (Fig. 10). It has been observed that the embedment of dopamine functionalized BNNTs (BNNTs@PDOPA) effectively reduces the CTE of PVDF as compared to pristine-BNNT/PVDF nanocomposites (PVDF = 197.3 × 10−6 K−1; PVDF/BNNTs = 160 × 10−6 K−1; PVDF/BNNTs@PDOPA = 125 × 10−6 K−1). The present work is the first report on the CTE of functionalized BNNT/PVDF nanocomposites. These results show that the interactions of the BNNTs with PVDF polymer matrix result in the restricted polymer chain movements due to the efficient matrix interactions.1
 | ||
| Fig. 10 Thermal mechanical analysis results of pristine PVDF, PVDF/BNNTs and PVDF/BNNT@PDOPA nanocomposites. | ||
It is clearly evident that the well functionalized surface of the BNNTs results in the better ability of the nanocomposite to reduce the thermal expansion.
Conclusions
Bioinspired chemical functionalization is an effective way to modify different types of nanotubes and extend their applications. A facile approach based on bioinspired dopamine for surface functionalization of inert non-reactive BNNTs was developed in this work. It has been observed that dopamine reacts strongly with BNNTs in aqueous solution. The polydopamine could form a stable reactive layer that tightly attached on the BNNTs, which provides opportunities for further surface modification. The dopamine induced functionalization is effective for BNNTs because of stronger π–π interactions as well as interaction of the amino group with the BNNTs. The interaction of dopamine with BNNTs was characterized by different characterization methods such as FESEM, TEM, XRD, TGA, FTIR, and Raman spectroscopy. The results clearly demonstrate that the dopamine induced functionalization is a novel method for the successful functionalization of BNNTs with the advantages that functionalization can be carried out in water and the functionalized BNNTs are soluble/dispersible in a wide range of solvents leading to their aqueous and/or organic solubility. The modulus and strength of the nanocomposites prepared from the functionalized nanotubes increase to significant extents as compared to the pristine PVDF matrix and pristine-BNNT/PVDF composites. The CTEs of the nanocomposites were also remarkably reduced. This indicates that the polymer-chain mobility decreases due to efficient matrix interactions with the embedded BNNTs. Hence the overall results of the present work suggest that the solubilization via bioinspired functionalization adds a new dimension in the applications of BNNTs for their unique properties.Acknowledgements
M. F. Lin and R. Sim acknowledge the research scholarships supported by Nanyang Technological University and Global Foundries Ltd. Singapore, respectively.Notes and references
- C. Zhi, Y. Bando, T. Terao, C. Tang, H. Kuwahara and D. Golberg, Adv. Funct. Mater., 2009, 19, 1857 CrossRef CAS.
- N. G. Chopra, R. J. Luyken, K. Cherreyetal, V. H. Crespi, M. L. Cohen, S. G. Louie and A. Zettl, Science, 1995, 269, 966 CAS.
- E. Bengu and L. D. Marks, Phys. Rev. Lett., 2001, 86, 2385 CrossRef CAS.
- R. Arenal, O. Stephan, J.-L. Cochon and A. Loiseau, J. Am. Chem. Soc., 2000, 129, 16183 CrossRef.
- S. Velayudham, Y. K. Yap, C. H. Lee, S. A. Green, M. Xie, H. Liu, D. Blair and N. Bauman, ACS Appl. Mater. Interfaces, 2010, 4, 104 Search PubMed.
- C. Tang, Y. Bando, T. Sato and H. Kurashima, Chem. Commun., 2002, 1290 RSC.
- J. Wang, V. K. Kayastha, Y. K. Yap, J. Y. Fan, J. G. Lu, Z. W. Pan, I. N. Ivanov, A. A. Puretzky and D. B. Geohegan, Nano Lett., 2005, 12, 2528 CrossRef.
- S. K. Samanta, A. Pal, S. Bhattacharya and C. N. R. Rao, J. Mater. Chem., 2010, 20, 6881 RSC.
- A. Pal, B. S. Chhikara, A. Govindaraj, S. Bhattacharya and C. N. R. Rao, J. Mater. Chem., 2008, 18, 2593 RSC.
- R. Tenne and M. Redlich, Chem. Soc. Rev., 2010, 39, 1423 RSC.
- S. K. Samanta, A. Gomathi, S. Bhattacharya and C. N. R. Rao, Langmuir, 2010, 26, 12230 CrossRef CAS.
- G. Gou, B. Pan and L. Shi, ACS Nano, 2010, 4, 1313 CrossRef CAS.
- W.-Q. Han and A. Zettl, J. Am. Chem. Soc., 2003, 125, 2062 CrossRef CAS.
- F. L. Cao, W. Ren, X. Y. Xu, Y. M. Ji and C. Y. Zhao, Phys. Chem. Chem. Phys., 2009, 11, 6256 RSC.
- S. Pal, S. R. C. Vivekchand, A. Govindaraj and C. N. R. Rao, J. Mater. Chem., 2007, 17, 450 RSC.
- C. Zhi, Y. Bando, C. Tang, S. Honda, K. Sato, H. Kuwahara and D. Golberg, Angew. Chem., Int. Ed., 2005, 44, 7932 CrossRef CAS.
- T. Sainsbury, T. Ikuno, D. Okawa, D. Pacile, J. M. J. Frechet and A. Zettl, J. Phys. Chem. C, 2007, 111, 12992 CAS.
- A. Maguer, E. Leroy, L. Bresson, E. Doris, A. Loiseau and C. Mioskowski, J. Mater. Chem., 2009, 19, 1271 RSC.
- Y. Li, Z. Zhou and J. Zhao, Nanotechnology, 2008, 19, 015202 CrossRef.
- C. Zhi, Y. Bando, C. Tang, R. Xie, T. Sekiguchi and D. Golberg, J. Am. Chem. Soc., 2005, 127, 15996 CrossRef CAS.
- S.-Y. Xie, W. Wang, K. A. S. Fernando, X. Wang, Y. Lin and Y.-P. Sun, Chem. Commun., 2005, 3670 RSC.
- W. Wang, Y. Bando, C. Zhi, W. Fu, E. Wang and D. Golberg, J. Am. Chem. Soc., 2008, 130, 8144 CrossRef CAS.
- J. Yu, Y. Chen and B. M. Cheng, Solid State Commun., 2009, 149, 763 CrossRef CAS.
- T. Terao, Y. Bando, M. Mitome, C. Zhi, C. C. Tang and D. Golberg, J. Phys. Chem. C, 2009, 113, 13605 CAS.
- V. K. Thakur, E. J. Tan, M. F. Lin and P. S. Lee, J. Mater. Chem., 2011, 21, 3751 RSC.
- V. K. Thakur, E. J. Tan, M. F. Lin and P. S. Lee, Polym. Chem., 2011, 2, 2000 RSC.
- M. F. Lin, V. K. Thakur, E. J. Tan and P. S. Lee, RSC Adv., 2011, 1, 576 RSC.
- M. F. Lin, V. K. Thakur, E. J. Tan and P. S. Lee, J. Mater. Chem., 2011, 21, 16500 RSC.
- H. Lee, S. M. Dellatore, W. M. Miller and P. B. Messersmith, Science, 2007, 318, 426 CrossRef CAS.
- J. Ryu, S. H. Ku, H. Lee and C. B. Park, Adv. Funct. Mater., 2010, 20, 2132 CrossRef CAS.
- V. K. Thakur, M. F. Lin, E. J. Tan and P. S. Lee, J. Mater. Chem., 2012 10.1039/c2jm15665b.
- X. Fan, L. Lin and P. B. Messersmith, Compos. Sci. Technol., 2006, 66, 1195 Search PubMed.
- C. Zhi, Y. Bando, C. Tang, H. Kuwahara and D. Golberg, Chem.–Asian J., 2009, 4, 1536 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and characterization techniques of the BNNTs. See DOI: 10.1039/c2py00612j |
| This journal is © The Royal Society of Chemistry 2012 |

